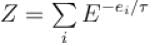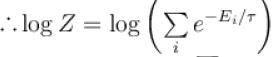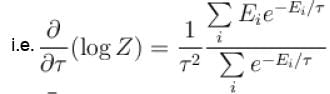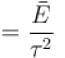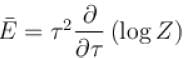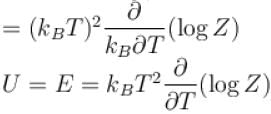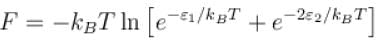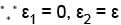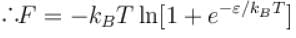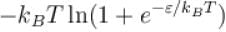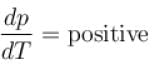Thermodynamic Potential MCQ Level – 2 - IIT JAM MCQ
10 Questions MCQ Test - Thermodynamic Potential MCQ Level – 2
In a pressure cooker, the vapour pressure is greater than 1 atm. Under such condition, the boiling temperature of water will be
Select one:
Select one:
On a p-T graph, the curve which connects points at which vapour (v) and solid (s) exists in equilibrium called
Select one:
Select one:
In one of the Maxwell’s relations 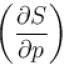 equals
equals
Select one:
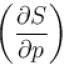 equals
equalsSelect one:
Entropy of a system having average energy E and partition function Z is defined as
Select one:
Helmholtz free energy, F = U – TS. Which relation holds true? (where U = Internal energy; S = Entropy)
Select one:
Which of the following is true with regards to critical point?
Select one:
Helmholtz free energy function and internal energy U are related as
Select one:
Which of the following relations between internal energy U and the canonical partition function Z is true?
Select one:
Consider two level question system with energies ε1 = 0, ε2 = ε, The Helmholtz free energy of the system is given by
Select one:
The boiling point of water will decrease as we go to a higher altitude because
Select one:


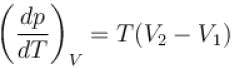
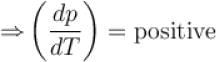

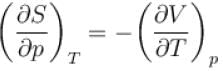
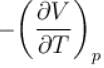

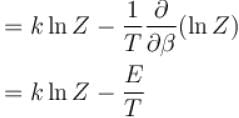
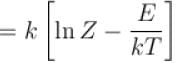
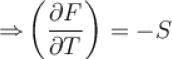
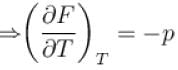
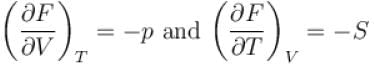
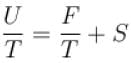
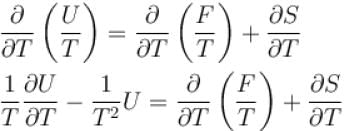
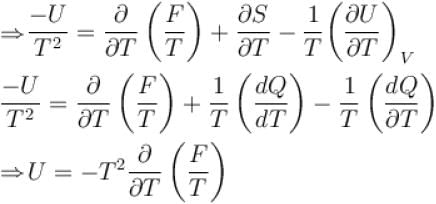
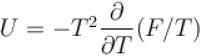
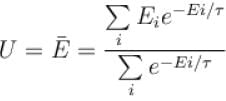
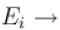 Energy of the system in the ith state the partition function
Energy of the system in the ith state the partition function