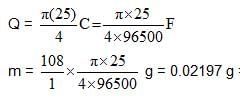UP PGT Chemistry Mock Test - 2 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 2
Direction (Q. Nos. 26) Choice for the correct combination of elements from coloumn I and Coloumn II are given as option (a), (b), (c) and (d) out of which one is correct.
Match the species in Column I with their properties in Column II.
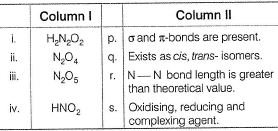

Match the species in Column I with their properties in Column II.
Of the following sets which one does NOT contain isoelectronic species ? [AIEEE- 2005]
Fluorobenzene (C6H5F) can be synthesized in the laboratory -
[AIEEE 2006]
pH of an aqueous solution of NaCl at 85°C should be
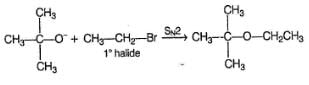
Which is the best reaction for preparation of t-butyl ethyl ether?
Direction (Q. Nos. 1-18) This section contains 18 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The correct statement regarding a chiral compound is
During electrolysis of acidified water ,O2 gas is formed at the anode. To produce O2 gas at the anode at the rate of 0.224 cc per second at STP,current passed is
In which of the following pairs of molecules/ions both the species are not likely to exist?
What is the equilibrium constant for the reaction P4(s) + 5O2(g) 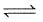 P4O10(s) :
P4O10(s) :
Three cell A, B and C has equilibrium constant in the ratio 1:4 : 9 respectively. Arrange the following cells in the order of increasing Gibbs free energy.
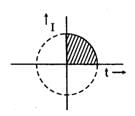
With t taken in seconds and I taken in Amp, the variation of I follows the equation
t2 + I2 = 25
what amount of Ag will be electrodeposited with this current flowing in the interval 0-5 second ? (Ag : 108)
Select the set of compounds having fractional oxidation number in one or more atoms.
Which of these ions Cu+, Co3+, Fe2+ is stable in aqueous medium.
Given :
E°Cu2+/Cu+ = 0.15 volt ; E°Cu+/Cu = 0.53 V ; E°Co3+/Co2+ = 1.82 V ;
E°Fe3+/Fe2+ = 0.77 V ; E°Fe2+Fe = - 0.44 V ; E°O2,H+/H2O = 1.23 V
If X is a nonmetal, its oxide X2O3 is expected to be a/an ______ oxide.
Which reaction, with the following values of ΔH and ΔS at 400 K is spontaneous and endothermic?
Optical rotation of a newly synthesised chiral compound is found to be +60°. Which of the following experiment can be performed to establish that optical rotation is not actually -300°?
The ionization energy of the lowest state, also called as the ground state, is:
The combination of a carbonyl group and a hydroxyl group on the same carbon atom is called a __________ group.
The element ____________ is also known as Unnilunium.
For the following gases equilibrium. N2O4(g) 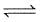 2NO2(g) Kp is found to be equal to Kc. This is attained when temperature is
2NO2(g) Kp is found to be equal to Kc. This is attained when temperature is
At 25 ° C, the density of 18 MH2SO4 is 1.8 g cm3. Thus, mass percentage of H2SO4 in aqueous solution is
Direction (Q. Nos. 21-25) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
Passage I
Electronegativity values (EN) of elements have been given (in Pauling’s scale)
Graph shows variation of percentage ionic character with (EN) difference of two elements.
Q. Which of the following bonds is most polar?
For which of the following reaction the units of rate constant and rate of the reaction are same?
Radius of Bohr’s orbit of H-atom is 52.9 pm. An emission in H-atom starts from the orbit having radius 1.3225 nm and ends at 211.6 pm.
Q. Spectral line appears in .......... region.
In balancing the half-reaction, CN- → CNO-
The number of electrons that must be added is
Consider the following set of molecules.
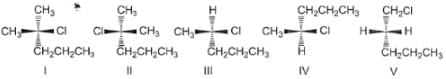
The pairs of enantiomers are
|
30 tests
|