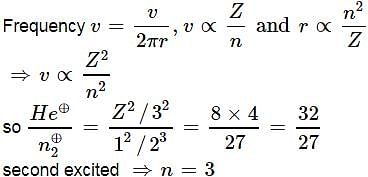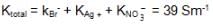UP PGT Chemistry Mock Test - 5 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 5
A fresh precipitate can be transformed into colloidal sol by:
At 675 K, H2(g) and CO2(g) react to form CO(g) and H2O(g), Kp for the reaction is 0.16. If a mixture of 0.25 mole of H2(g) and 0.25 mol of CO2 is heated at 675 K, mole% of CO(g) in equilibrium mixture is :
The total number of tetrahedral voids in the face-centred unit cell is
Butylated – Hydroxy Toluene is added in packed foods to preserve fats because
Which transition metal oxide has appearance and conductivity like that of copper?
Which is the most suitable reagent for the following transformation?
Which one of the following is a water soluble vitamin?
How many distinct alkene products are possible when the alkyl iodide given below undergoes E2 elimination?
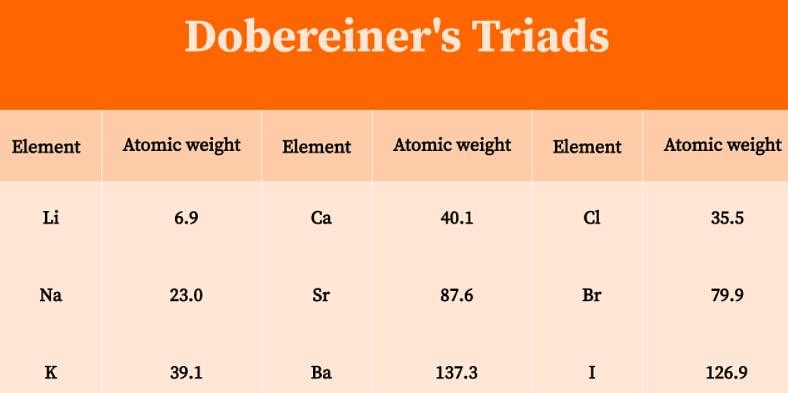
According to Dobereiner’s law of triads the number of elements present in each group is:
The Aufbau principle states : In the ground state of the atoms, the orbitals are filled in order of
What is the most efficient method to get water with zero degrees hardness?
An electron in H-atom in its ground state absorbs 1.5 times as much as energy as the minimum required for its escape from the atom
Q.
Thus, kinetic energy given to the emitted electron is
Calculate the cell EMF in mV for
Pt|H2(1atm) |HCl(0.01M)|AgCl(s)| Ag(s) at 298 K
If ΔG°r values are at 25°C
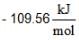 for AgCl(s) and
for AgCl(s) and 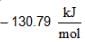 for H+ + Cl-) (aq)
for H+ + Cl-) (aq)
2, 3-dimethyl butaneWhich of the following anion will be migrates towards anode to prepare 2, 3-dimethyl butane in the given reaction.
An ion M2+, forms the complexes [M(H2O)6]2+, [M(en)3]2+ and [MBr6]4-, match the complex with the appropriate colour.
Ratio of frequency of revolution of electron in the second excited state of He⊕and second state of hydrogen is
The chemical reaction in which reactants require high amount of activation energy are generally
Which of the following does not show optical isomerism?
We have taken a saturated solution of AgBr.Ksp of AgBr is 12 x 10 – 14 . If 10 – 7 mole of AgNO3 are added to 1 litre of this solution then the conductivity of this solution in terms of 10 – 7 Sm – 1 units will be
[given 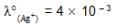 Sm2 mol-1
Sm2 mol-1 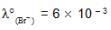 Sm2 mol-1, 5 x 10-3 Sm2mol-1]
Sm2 mol-1, 5 x 10-3 Sm2mol-1]
Why some of the physical properties of solids show different values when measured along different directions in the same crystals?
Which one of the following has the minimum boiling point?
[AIEEE-2004]
Hot carbon reacts with steam to produce an equimolar mixture of CO(g)and H2(g) known as water gas
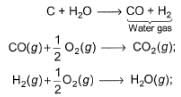
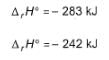
Q.
Energy released , if water gas is used as fuel is
Which of the following is thermally the most stable?
PCl5 dissociation a closed container as :
PCl5(g)  PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is α, the partial pressure of PCl3 will be :
|
30 tests
|