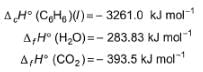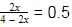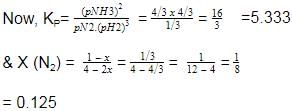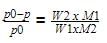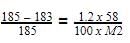UP PGT Chemistry Mock Test - 6 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 6
Among the following elements element with highest ionisation enthalpy is:
Which of the following elements are called representative elements?
Gadolinium belongs to 4f series. Its atomic number is 64. Which of the following is the correct electronic configuration of gadolinium?
A measured temperature is 1000F on Fahrenheit scale, then what is this reading be on Celsius scale:
Energy of the electron in nth orbit is given by E Wavelength of light required to excite an electron in an H-atom from level n = 1 to n = 2 will be (h = 6.62 x 10-34 J s ; c = 3.0 x 108ms -1)
[AIEEE 2012]
Oxygen exhibits +2 oxidation state in the compound:
pH of saturated solution of silver salt of monobasic acid HA is found to be 9.
Find the Ksp of sparingly soluble salt Ag A(s).
Given : Ka(HA) = 10-10
Calculate resonance energy of N2O from the following data
ΔfH° (N2O) = 82 kJ mol-1
The `milk of magnesia' used as an antacid is chemicaly -
At 700 K and 350 bar, a 1 : 3 mixture of N2(g) and H2(g) reacts to form an equilibrium mixture containing X (NH3)= 0.50. Assuming ideal behaviour Kp for the equilibrium reaction,
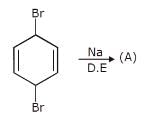
How many dichlorocyclohexane would be produced upon free radical chlorination of chlorocyclohexane?
As you move from left to right across the periodic table:
Mole fraction of C3H5(OH)3 in a solution of 36 gm of water and 46 gm of glycerine is
How many atoms of hydrogen are in 67.2 L of H2 at STP?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
A handbook gives the concentration of water in a 2.772 M aqueous solution of NaOH as 998.0 g L -1.
Q.
Molality of solution is
Study the following reactions.
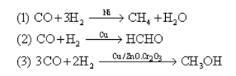
Which characteristic of catalyst is represented by them.?
The number of atoms in 0.1 mol of a triatomic gas is
Hydrogen has tendency to gain one electron to acquire helium configuration, in this respect it resembles:
Direction (Q. Nos. 18 and 19) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q. S2O32- has two types of sulphur atoms. What is the difference in the oxidation states of two types of sulphur atoms?
For an aqueous solution, freezing point is _0.186ºC . Boiling point of the same solution is
(Kƒ = 1.86º K mol-1 kg) and Kb = 0.512º K mol-1 kg)
[AIEEE-2002]
In the electrolysis of aqueous sodium chloride solution, two types of reactions can take place at anode :
I. 2Cl- (aq) → Cl2(g) +2e-
II. 2H2O(l)g → O2 (g) + 4H+(aq) + 4e-
Select the correct statement(s) about these.
Sodium pentacyanonitrosylferrate(II) is also called?
|
30 tests
|