UP PGT Chemistry Mock Test - 8 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 8
What is molarity in terms of volume strength of hydrogen peroxide?
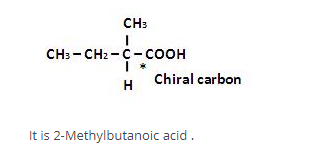
The number of optical isomers possible for 2, 3-pentanediol is:
The curve showing the variation of adsorption with pressure at constant temperature is known as
A group of 14 element is converted into n – type semiconductor by dopping it with
For the cell (at 298 K)
Ag(s) | AgCl(s) | Cl-(aq) || AgNO3(aq) | Ag(s)
Which of the following is correct –
Electron-rich hydrides has excess electrons that are present as
What are the roots of 1, 1, and 9 respectively as per the IUPAC nomenclature, and find out its symbol?
The kinetic energy of an electron in the second Bohr orbit of a hydrogen atom is (a0 is Bohr radius)
[AIEEE 2012]
What is the major product in the following reaction?
Change in volume of the system does not alter the number of moles in which of the following equilibrium
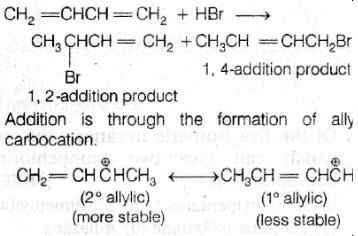
Reaction of one molecule of HBr with one molecule of 1,3–butadiene at 40ºC given predominantly
[AIEEE-2005]
Which among the following statement is not true for rate constant of a reaction?
From the following, pick out the potential energy profile for a SN1 reaction.
A definite amount of solid NH4HS is placed in a flask already containing ammonia gas at a certain temperature and 0.50 atm pressure. NH4HS decomposes to give NH3 and H2S and at equilibrium total pressure in flask is 0.84 atm. The equilibrium constant for the reaction is :
Following suborbits with values of n and l are given
Q. Increasing order of energy of these suborbits is
According to MO theory which of the following lists ranks the nitrogen species in terms of increasing bond order?
Which of the following is general electronic configuration of actinides?
A gas absorbs a photon of 355 nm and emits at two wavelengths. If one of the emissions is at 680 nm, the other is at:
In a reaction, A + B → Product, rate is doubled when the concentration of B is doubled, and rate increases by a factor of 8 when the concentrations of both the reactants (Aand B) are doubled, rate law for the reaction can be written as
Which one of the following configuration represents a metallic character?
|
30 tests
|