UP PGT Chemistry Mock Test - 9 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2025 - UP PGT Chemistry Mock Test - 9
AgNO3 sample is 85% by mass. To prepare 125 mL of 0.05 M AgNO3 solution, AgNO3 sample required is
An ionic compound A+B- is most like to be formed from A and B when
Which of the following could result as a product in the aldol condensation reaction?
Which one of the following shows oxidation state upto + 7?
Rate of reaction for the combustion of propane is equal to
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g)
Arrange NaCI, MgCI2, AICI3, SiCI4 in increasing solubility in ether (non-polar solvent).
What volume of hydrogen gas at 273 K and 1 atm pressure will be consumed in obtaining 21.6 g elemental boron (atomic mass = 10.8) from the reduction of boron trichloride by hydrogen?
Which among the following is an example of first order reaction?
The pKa of a weak acid, HA, is 4.80. The pKb of a weak base, BOH, is 4.78. The pH of an aqueous solution of the corresponding salt, BA, will be:
Comprehension Type
Direction (Q. Nos. 13-15) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Passage
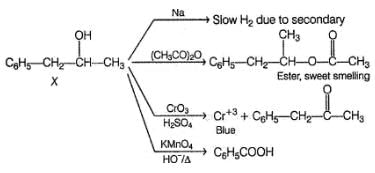
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
The structure of X is
In the catalyzed decomposition of benzene diazonium chloride,
Half life period is found to be independent of the initial concentration of the reactant. After 10 min, the volume of N2 gas collected is 10 L and after the reaction is complete, it is 50 L. Hence, the rate constant of the reaction(in min-1) is
A brown ring is formed in the ring test for NO3– ion. It is due to the formation of
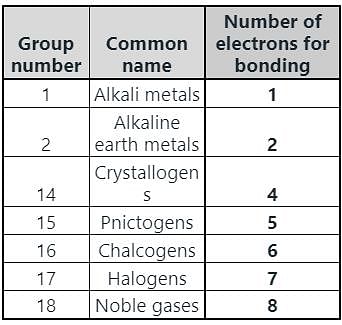
In which group of the modern periodic table are halogens placed?
Consider the cell Ag(s)|AgBr(s)|Br-(aq)||AgCl(s)|Cl-(aq)|Ag(s) at 25°C. The solubility product constants of AgBr & AgCl are respectively 5 X 10 – 13 & 1 X 10 – 10. For what ratio of the concentrations of Br- & Cl- ions would the emf of the cell be zero ?
A compound Mp Xq has cubic close packing arrangement of X. Its unit cell structure is shown below. The empirical formula of the compound is
Given, BE (H—H) - x1, BE = x2, BE (O—H) = x3 and for H2O (l) → H2O (g), ΔH = x4 mol-1, then ΔfH° [H2O (l)] is
Among the following species the pair that have V-shaped geometry is:
Select the correct statement(s) about electrolysis of aqueous CuSO4 solution.
Alkyl halides react with dialkyl copper reagents to give
[AIEEE-2005]
Equilibrium constant for the reaction,
is 1.8 x 109. Hence, equilibrium constant for
Aqueous solution of barium phosphate which is 100% ionised has ΔTf / Kf as 0.40. Hence, given solution is x * 10-2 molal. What is the value of x?
Which of the following option with respect to the Pauling electronegativity values of the element is
|
30 tests
|



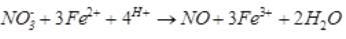
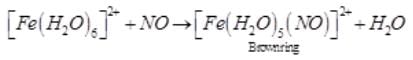
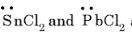 are bent shape molecule.
are bent shape molecule.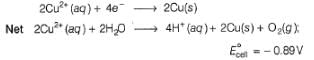 since, E0cell < 0, in electrolysis.
since, E0cell < 0, in electrolysis.



















