VITEEE Chemistry Test - 6 - JEE MCQ
30 Questions MCQ Test - VITEEE Chemistry Test - 6
Which of the following process is employed to convert alkyl halide into alcohol?
Which of the following compounds will undergo self aldol condensation in the presence of cold dilute alkali?
An organic compound 'A' has the molecular formula C₃H₆O. It undergoes iodoform test. When saturated with HCl it gives 'B' of molecular formula C₉H₁₄O. 'A' and 'B' respectively are
Irrespective of the source, pure sample of water always yields 88.89% mass of oxygen and 11.11% mass of hydrogen. This is explained by the law of
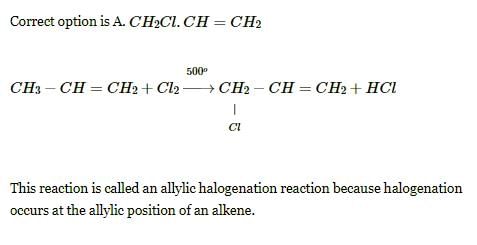
How many monochlorobutanes will be obtained on chlorination of n-butane?
Which one of the following esters is obtained by the esterification of propan-2-ol with ethanoic acid?
A is a higher phenol and B is an aromatic carboxylic acid. Separation of a mixture of A and B can be carried out easily by having a solution
A chemical reaction is catalyzed by a catalyst X. Hence X
A glass bulb is filled with NO₂ gas and immersed in an ice bath at 0oC which becomes colourless after some time . This colourless gas will be
The Δ H value for the reaction H2 + Cl2 → 2HCl is − 44.12 K.cal. If E1 is the activation energy of the reactants and E2 is the activation energy of the products, then for the above reaction
Calculate the heat of formation Δ H of CO (in kcal) from the following data :
C(graphite) + O2 (g) → CO2 (g) ; Δ H = − 94 K . c a l
CO(g) + 1/2O2 (g) → CO2 (g) ; Δ H = − 68 K . c a l
The pair in which both species have the same magnetic moment (spin only value) is :
When benzenediazonium chloride in hydrochloric acid reacts with cuprous chloride, then chlorobenzene is formed. The reaction is called
96500 C electricity is passed through CuSO₄. The amount of copper precipitated is
The element, which forms oxides in all oxidation states + 1 to + 5, is
Acetic anhydride reacts with diethyl ether in the presence of anhydrous AlCl₃ to give
Which of the following metal is leached by cyanide process?
At which one of the following temperature-pressure conditions, the deviation of a gas from ideal behaviour is expected to be minimum ?