WBJEE Chemistry Test - 6 - JEE MCQ
30 Questions MCQ Test - WBJEE Chemistry Test - 6
Which of the following does not turn Schiff's reagent to pink ?
The energy of second Bohr orbit of the hydrogen atom is -328KJ mol⁻1. The energy of fourth Bohr orbit would be
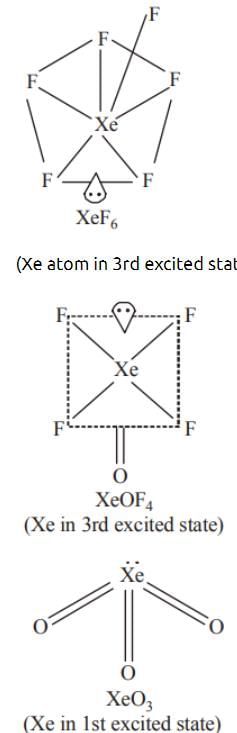
Among the molecules (a)XeO₃ (b)XeOF (c)XeOF₆,those having same no. of lone pairs on Xe are
The heat of formation of CO₂ is -94 Kcal mol⁻1 whereas ∆H for 2CeO₂ → 2CO is -110.5 Kcal,The enthalpy of combustion carbon is
The equilibrium constant for the following reaction will be 3 A + 2 B → C
A reaction with respect to the reactant A has a rate constant of 5 s⁻1. If we start with[A]= 10 mol-L⁻1, then in what time the concentration of A becomes 1.0 mol-L⁻1?
In an electrochemical cell, if Eº is the EMF of the cell involving 'n' mole of electrons, then ∆Gº is
The radioisotope, tritium (3H₁) has a half-life of 12.3 years. If the initial amount of tritium is 32 mg, how many miligrams of it would remain after 49.2 years ?
1. Isomers which are mirror images of each other are called enantiomers
2. A mixture of equal parts of enantiomers is called a racemic modification
3. Enantiomers show different properties-physical or chemical -only in a chiral medium
4. A racemic modification is converted by an optically active agent into mixture of diasteromers that can be separated.
Which of the above statements are correct ?