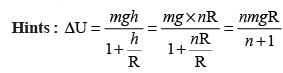WBJEE Previous Year (2010) - JEE MCQ
30 Questions MCQ Test - WBJEE Previous Year (2010)
Experimental investigations show that the intensity of solar radiation is maximum for a wavelength 480 nm in the visible region. Estimate the surface temperature of sun. Given Wein’s constant b = 2.88 × 10–3 mK.
The temperature of an ideal gas is increased from 120 K to 480 K. If at 120 K, the root mean square speed of gas molecules is v, then at 480 K it will be
Two mirrors at an angle θº produce 5 images of a point. The number of images produced when θ is decreased to θº – 30º is
he radius of the light circle observed by a fish at a depth of 12 meter is (refractive index of water = 4/3)
In Young’s double slit experiment, the fringe width is β. If the entire arrangement is placed in a liquid of refractive index n, the fringe width becomes :
A plano-convex lens (f = 20 cm) is silvered at plane surface. Now focal length will be :
The light beams of intensities in the ratio of 9 : 1 are allowed to interfere. What will be the ratio of the intensities of maxima and minima ?
If x1 be the size of the magnified image and x2 the size of the diminished image in Lens Displacement Method, then the size of the object is :
A point charge +q is placed at the centre of a cube of side L. The electric flux emerging from the cube is
Two capacitors of equal capacity are first connected in parallel and then in series. The ratio of the total capacities in the two cases will be
n identical droplets are charged to v volt each. If they coalesce to form a single drop, then its potential will be
The reading of the ammeter in the following figure will be
A wire of resistance R is elongated n-fold to make a new uniform wire. The resistance of new wire
The ratio of magnetic field and magnetic moment at the centre of a current carrying circular loop is x. When both the current and radius is doubled the ratio will be
The current through a coil of self inductance L = 2mH is given by I = t2 e–t at time t. How long it will take to make the e.m.f. zero?
The magnetic flux through a loop of resistance 10 Ω is given by φ = 5t2 – 4t + 1 Weber. How much current is induced in the loop after 0.2 sec ?
The decimal equivalent of the binary number (11010.101)2 is
In a common emitter configuration, a transistor has β = 50 and input resistance 1 kΩ. If the peak value of a.c. input is 0.01 V then the peak value of collector current is
Half-life of a radioactive substance is 20 minute. The time between 20% and 80% decay will be :
The energy released by the fission of one uranium atom is 200 MeV. The number of fissions per second required to produce 3.2 W of power is (Take 1 eV = 1.6 × 10–19 J)
A body is projected with a speed u m/s at an angle β with the horizontal. The kinetic energy at the highest point is 3/4th of the initial kinetic energy. The value of β is :
A ball is projected horizontally with a velocity of 5 m/s from the top of a building 19.6 m high. How long will the ball take of hit the ground ?
A stone falls freely from rest and the total distance covered by it in the last second of its motion equals the distance covered by it in the first three seconds of its motion. The stone remains in the air for
Two blocks of 2 kg and 1 kg are in contact on a frictionless table. If a force of 3 N is applied on 2 kg block, then the force ofcontact between the two blocks will be :
If momentum is increased by 20%, then kinetic energy increases by
A boy of mass 40 kg is climbing a vertical pole at a constant speed. If the coefficient of friction between his palms and the poleis 0.8 and g = 10 m/s2, the horizontal force that he is applying on the pole is
The value of ‘λ’ for which the two vectors a =5iˆ+λ jˆ+kˆ and b =iˆ−2jˆ+kˆare perpendicular to each other is
The height vertically above the earth’s surface at which the acceleration due to gravity becomes 1% of its value at the surface is (R is the radius of the Earth)
The change in the gravitational potential energy when a body of mass m is raised to a height nR above the surface of the Earth is (here R is the radius of the Earth)