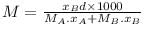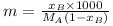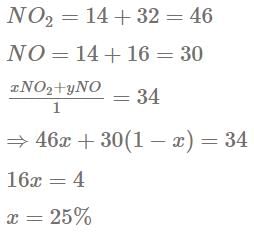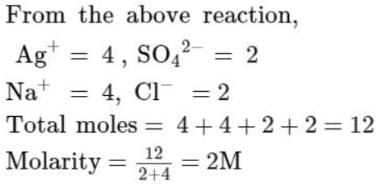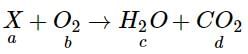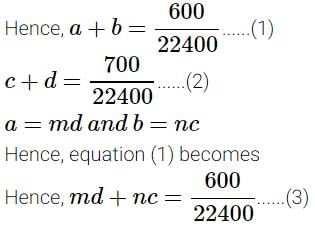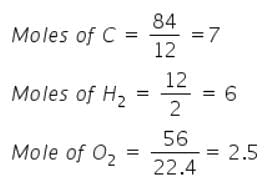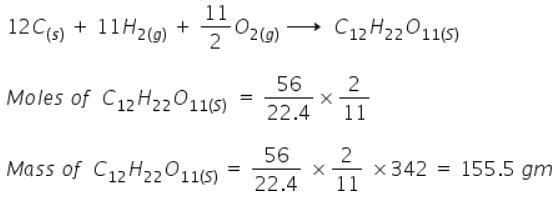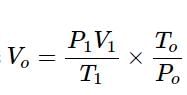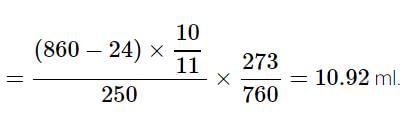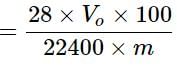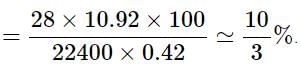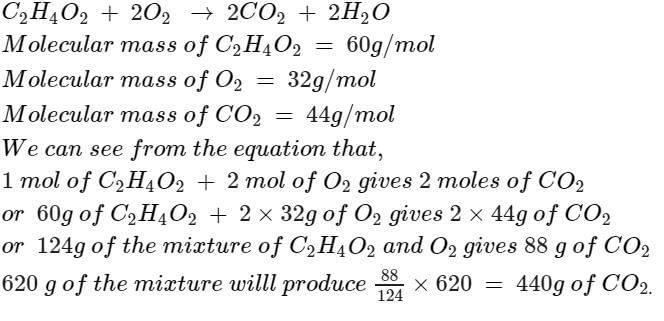Test: Stoichiometry - JEE MCQ
30 Questions MCQ Test - Test: Stoichiometry
For the reaction 2x + 3y + 4z → 5w
Initially if 1 mole of x, 3 mole of y and 4 mole of z is taken. If 1.25 mole of w is obtained then % yield of this reaction is
A solution of A (MM = 20 ) and B (MM =10), [Mole fraction XB = 0.6] having density 0.7 gm/ml then molarity and molality of B in this solution will be _____ and _____ respectively.
125ml of 8% w/w NaOH solution (sp. gravity 1) is added to 125 ml of 10% w/v HCl solution. The nature of resultant solution would be _____
Ratio of masses of H2SO4 and Al2(SO4)3 is grams each containing 32 grams of S is _____
The vapour density of a mixture of gas A (Molecular mass = 40) and gas B (Molecular mass = 80) is 25. Then mole % of gas B in the mixture would be
For the reaction 2A + 3B + 5C → 3D
Initially if 2 mole of A, 4 mole of B and 6 mole of C is taken, with 25% yield, moles of D which can be produced are _________
Two elements X (atomic mass = 75) and Y (atomic mass = 16) combine to give a compound having 75.8% of X. The formula of the compound is :
Equal volumes of 10% (v/v) of HCl is mixed with 10% (v/v) NaOH solution. If density of pure NaOH is 1.5 times that of pure HCl then the resultant solution be :
10 ml of a compound containing 'N' & 'O' is mixed with 30 ml of H2 to produce H20(l) and 10 ml of N2(g). Molecular formula of compound if both reactants react completely, is
The percentage by mole of NO2 in a mixture NO2(g) and NO (g) having average molecular mass 34 is :
Assuming complete precipitation of AgCl, calculate the sum of the molar concentration of all the ions if 2 lit of 2M Ag2SO4 is mixed with 4 lit of 1 M NaCl solution is :
What volumes should you mix of 0.2M NaCl and 0.1M CaCl2 solution soo that in resulting solution the concentration of positive ion is 40% lesser than concentration of negative ion. Assuming total volume of solution 1000ml.
Weight of oxygen in Fe2O3 and FeO is in the simple ratio for the same amount of iron is :
The number of atoms present in 0.5 g-atoms of nitrogen is same as the atoms in
The oxide of a metal contains 30% oxygen by weight. If the atomic ratio of metal and oxygen is 2 : 3, determine the atomic weight of metal.
The O18/O16 ratio in some meteorites is greater than that used to calculate the average atomic mass of oxygen on earth. The average mass of an atom of oxygen in these meteorites is _____ that of a terrestrial oxygen atom?
Mass of one atom of the element A is 3.9854 x 10-23 . How many atoms are contained in 1g of the element A?
The density of quartz mineral was determined by adding a weighed piece to a graduated cylinder containing 51.2ml water. After the quartz was submersed, the water level was 65.7 ml. The quartz piece weighed 38.4g. What was the density of quartz?
A sample of clay contains 40% sillica and 15% water. The sample is partially dried by which it loses 5 gm water. If the percentage of water in the partially dried clay is 8, calculate the percentage of silica in the partially dried clay.
A definite amount of gaseous hydrocarbon was burnt with just sufficient amount of O2. The volume of all reactants was 600 ml, after the explosion the volume of the products [CO2(g) and H2O(g)] was found to be 700 ml under the similar conditions. The molecular formula of the compound is :
One gram is silver salt of an organic dibasic acid yields , on strong heating , 0.5934 g of silver. if the weight percentage of carbon in it 8 times the weight percentage of hydrogen and half the weight percentage of oxygen, determine the molecular formula of the acid. [Atomic weight of Ag = 108]
C6H5OH(g) + O2(g) → CO2(g) + H2O(/)
Magnitude of volume change if 30 ml of C6H5OH (g) is burnt with excess amount of oxygen, is
Similar of % labelling of oleum, a mixture of H3PO4 and P4O10 is labelling as ( 100 + x ) % where x is the maximum mass of water which can react with P4O10 present in 100 gm mixture of H3PO4 and P4O10. If such a mixture is labelled as 127% Mass of P4O10 is 100 gm of mixture, is
Mass of sucrose C12H22O11 produced by mixing 84 gm of carbon, 12 gm of hydrogen and 56 lit.O2 at 1 atm & 273 K according to given reaction, is C(s) + H 2(g) + O2(g) → C12H22O11(s)
If 50 gm oleum sample rated as 118% is mixed with 18 gm water, then the correct option is
In the quantitative determination of nitrogen using Duma’s method, N2 gas liberated from 0.42 gm of a sample of organic compound was collected over water. If the volume of N2 gas collected was 100/11 ml at total pressure 860 mm Hg at 250 K, % by mass of nitrogen in the organic compound is
[Aq. tension at 250K is 24 mm Hg and R = 0.08 L atm mol–1 K–1]
40 gm of a carbonate of an alkali metal or alkaline earth metal containing some inert impurities was made to react with excess HCl solution. The liberated CO2 occupied 12.315 lit. at 1 atm & 300 K. The correct option is
The minimum mass of mixture of A2 and B4 required to produce at least 1 kg of each product is:
(Given At. mass of ‘A’ = 10; At mass of ‘B’ = 120) 5A2 + 2B4 → 2AB2 + 4A2B
The mass of CO2 produced from 620 gm mixture of C2H4O2 & O2, prepared to produce maximum energy is