NTSE Level Test: Chemical Reactions & Equations - Class 10 MCQ
20 Questions MCQ Test - NTSE Level Test: Chemical Reactions & Equations
2AgI(s)  2Ag(s) + I2(g) The colour of iodine is
2Ag(s) + I2(g) The colour of iodine is
 2Ag(s) + I2(g) The colour of iodine is
2Ag(s) + I2(g) The colour of iodine isWhat is the number of iron atoms and hydrogen atoms in the balanced equation for the reaction of steam on iron ?
MnO2 + 4HCI→ MnCl2 + H2O + Cl2 The oxidising agent is
The shiny finish to the walls is because of
Zinc reacts with silver nitrate to form which compounds?
On heating ferrous sulphate crystals, we obtain
The brown gas evolved on heating of copper nitrate is
Which of the following are exothermic processes ?
(i) Reaction of water with quick lime
(ii) Dilution of an acid
(iii) Evaporation of water
(iv) Sublimation of camphor (crystals)
In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
The reaction 4NH3 (g) + 5O2(g) → 4NO(g) + 6H2O(g) is an example of a
(i) Displacement reaction.
(ii) Combination reaction.
(iii) Redox reaction.
(iv) Neutralisation reaction.
Barium chloride on reacting with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction
Which of the following is (are) an endothermic process(es)?
(i) Dilution of sulphuric acid
(ii) Sublimation of dry ice
(iii) Condensation of water vapours
(iv) Evaporation of water
The following reaction is used for the preparation of oxygen gas in the laboratory
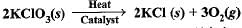
Which of the following statement (s) is (are) correct about the reaction ?
Solid calcium oxide reacts vigorously with water to form calcium hydroxide accompanied by liberation of heat. This process is called slaking of lime. Calcium hydroxide dissolves in water to form its solution called lime water. Which among the following is (are) true about slaking of lime and the solution formed ?
(i) It is an endothermic reaction.
(ii) It is an exothermic reaction.
(iii) The pH of the resulting solution will be more than seven.
(iv) The pH of the resulting solution will be less than seven.
Which of the following gases can be used for storage of fresh sample of an oil for a long time?
Which among the following is (are) double displacement reaction(s) ?
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2SO4 + BaCl2 → BaSO4 + 2NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2O2 →CO2 + 2H2O
Electrolysis of water is a decomposition reaction. The molar ratio of hydrogen and oxygen gases liberated during electrolysis of water is
Three beakers labelled as A, B and C each containing 25 ml of water were taken. A small amount of NaOH, anhydrous CuSO4 and NaCl were added to the beakers A, B and C respectively. It was observed that there was an increase in the temperature of the solutions contained in beakers A and B, whereas in case of beaker C, the temperature of the solution falls. Which one of the following statement(s) is (are) correct ?
(i) In beakers A and B, exothermic process has occurred
(ii) In beakers A and B, endothermic process has occurred
(iii) In beaker C exothermic process has occurred
(iv) In beaker C endothermic process has occurred
Which one of the following processes involve chemical reactions ?



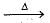 2CuO + 4NO2 + O2
2CuO + 4NO2 + O2















