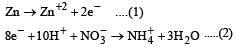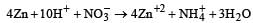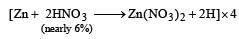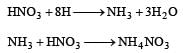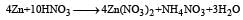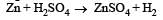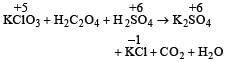31 Year NEET Previous Year Questions: Redox Reactions - NEET MCQ
16 Questions MCQ Test Chemistry Class 11 - 31 Year NEET Previous Year Questions: Redox Reactions
Which substance serves as reducing agent in the following reaction ? [1994]
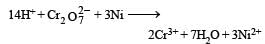
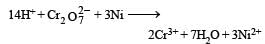
Phosphorus has the oxidation state of + 3 in
In which of the following reactions, there is no change in valency ? [1994]
The oxidation number of chromium in potassium dichromate is [1995]
The loss of electron is termed as [1995]
The oxide, which cannot act as a reducing agent, is[1995]
Which of the following involves a redox reaction?
The oxidation number of phosphorus in pyrophosphoric acid is [1999]
The following redox reaction is balanced by which set of coefficients ? [1999]
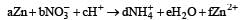

A compound contains atoms of three elements A, B and C. If the oxidation number of A is +2, B is +5, and that of C is –2, the possible formula of the compound is : [2000]
Zn gives H2 gas with H2SO4 and HCl but not with HNO3 because [2002]
The oxidation states of sulphur in the anions SO32–, S2O42– and S2O62– follow the order [2003]
Oxidation numbers of P in PO43– , of S in SO42– and that of Cr in Cr2O72– are respectively [2009]
When Cl2 gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from : [2012]
A mixture of potassium chlorate, oxalic acid and sulphuric acid is heated. During the reaction which element undergoes maximum change in the oxidation number ? [2012]
Standard reduction potentials of the half reactions are given below :
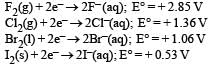
The strongest oxidising and reducing agents respectively are : [2012 M]
|
127 videos|245 docs|87 tests
|



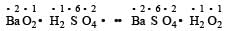
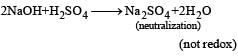
 (not redox reaction)
(not redox reaction)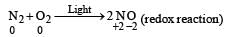
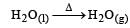 (not redox reaction)
(not redox reaction)