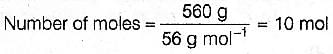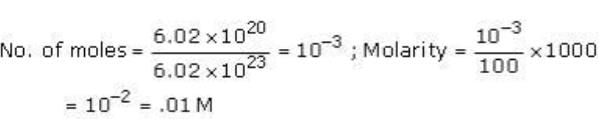Test: JEE Previous Year Questions- Stoichiometry - JEE MCQ
Test Description
6 Questions MCQ Test - Test: JEE Previous Year Questions- Stoichiometry
Test: JEE Previous Year Questions- Stoichiometry for JEE 2025 is part of JEE preparation. The Test: JEE Previous Year Questions- Stoichiometry questions and answers have been prepared
according to the JEE exam syllabus.The Test: JEE Previous Year Questions- Stoichiometry MCQs are made for JEE 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: JEE Previous Year Questions- Stoichiometry below.
Solutions of Test: JEE Previous Year Questions- Stoichiometry questions in English are available as part of our course for JEE & Test: JEE Previous Year Questions- Stoichiometry solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: JEE Previous Year Questions- Stoichiometry | 6 questions in 10 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Test: JEE Previous Year Questions- Stoichiometry - Question 1
The weight of 2.01 × 1023 molecules of CO is-
[AIEEE-2002]
Detailed Solution for Test: JEE Previous Year Questions- Stoichiometry - Question 1
Test: JEE Previous Year Questions- Stoichiometry - Question 2
In an organic compound of molar mass 108 gm mol-1 C, H and N atoms are present in 9 : 1 : 3.5 by weight. Molecular formula can be
[AIEEE-2002]
Detailed Solution for Test: JEE Previous Year Questions- Stoichiometry - Question 2
Test: JEE Previous Year Questions- Stoichiometry - Question 3
Number of atoms in 560 gm of Fe (atomic mass 56 g mol-1) is -
[AIEEE-2003]
Detailed Solution for Test: JEE Previous Year Questions- Stoichiometry - Question 3
Test: JEE Previous Year Questions- Stoichiometry - Question 4
6.02 ×1020 molecules of urea are present in 100 ml of its solution. The concentration of urea solution is -
(Avogadro constant, NA = 6.02 ×1023 mol-1)
[AIEEE-2004]
Detailed Solution for Test: JEE Previous Year Questions- Stoichiometry - Question 4
Test: JEE Previous Year Questions- Stoichiometry - Question 5
How many moles of magnesium phosphate, Mg3(PO4)2 will contain 0.25 mole of oxygen atoms ?
[AIEEE 2006]
Detailed Solution for Test: JEE Previous Year Questions- Stoichiometry - Question 5
Test: JEE Previous Year Questions- Stoichiometry - Question 6
In the reaction,
2Al(s)+6HCl(aq) → 2Al 3+(aq)+6Cl-(aq)+3H2 (g),
[AIEEE 2007]
Detailed Solution for Test: JEE Previous Year Questions- Stoichiometry - Question 6
Information about Test: JEE Previous Year Questions- Stoichiometry Page
In this test you can find the Exam questions for Test: JEE Previous Year Questions- Stoichiometry solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: JEE Previous Year Questions- Stoichiometry, EduRev gives you an ample number of Online tests for practice
Download as PDF