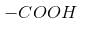Bihar STET Paper 2 Chemistry Mock Test - 1 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar STET Paper 2 Chemistry Mock Test - 1
Solubility of CuS(l), ZnS (II) and Na2S (III) in water is in the order
For macromolecules to form, one more of the following criteria are essential
Which of the following can be termed as mixed complex?
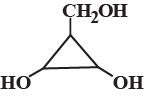
The number of optically active optical isomers of the compound is:
100 mL of aqueous solution of 0.01 M CaCI2 is evaporated to dryness when 0.15 g of residue is obtained. Thus, impurity present is
One mole of N2O4(g) at 300 K is left in a closed container under one atm. It is heated to 600 K when 20% by mass of N2O4 (g) decomposes to NO2(g). The resultant pressure is :
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Consider the following reaction,
CH2 = CH2 + Cl2 - H2O → ClCH2 — CH2OH  HOCH2CH2OH
HOCH2CH2OH
Q.
The most appropriate reagent X for the above reaction is
Direction (Q, Nos. 1 - 5) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which molecule will give the following dicarboxylic acid upon treatment with acidic solution of KMnO4?
Which among the following has square pyramidal geometry?
Direction (Q. Nos. 1-15) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. For the
I. benzene (C6H6) and
II. borazine (B3N3H6)
Select the correct statement.
Direction (Q. Nos. 1 - 11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. When light is shined on a mixture of chlorine and ethane, chloroethane is formed besides dichloroethane, trichloroethane and several other products. What reaction condition can optimise the yield of chloroethane?
Arrange the following compounds in decreasing order of their acid strength: i) trichloroacetic acid ii) trifluoroacetic acid iii) acetic acid and iv) formic acid
Which element acts as strong reducing agent?
How many gm of solid NaOH must be added to 100 ml of a buffer solution which is 0.1 M each w.r.t. Acid HA and salt Na+ A- to make the pH of solution 5.5. Given pKa(HA) = 5 (Use antilog (0.5)= 3.16)
The hardest substance among the following is
How many atoms of Oxygen are there in 18g of water?
(Hint: Avogadro’s Number = 6.02 x 1023 atoms/mol)
Give the name of the following compound:
Direction (Q. Nos. 1-7) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
The hybridisation of N in solid state for N2O5 is
The rate equation for the reaction 2 A + B → C is found to be : rate = k [A] [B] . The correct statement in relation to this reaction is that the
[AIEEE-2004]
Which one of the following statement is false?
[AIEEE-2004]
Only One Option Correct Type
Direction (Q, Nos. 1-9) This section contains 9 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which of the following will give a racemic mixture on reduction with NaBH4 followed by acid work-up?
Which of the following is not an oxidising agent?
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
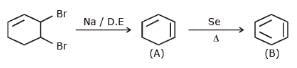
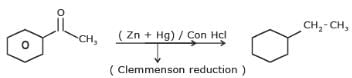
Q.
Identify the final major product of the reaction sequence.
Which among the following substances is an example of multimolecular colloids?
Which of the following is the primary agent of anticipatory socialization?
The following three aspects of intelligence are dealt with by Sternberg's triarchic theory except:
A portfolio is an assessment tool. It includes



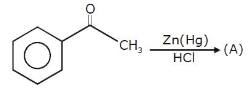

 - position.
- position.