NVS PGT Chemistry Mock Test - 4 - NVS TGT/PGT MCQ
30 Questions MCQ Test - NVS PGT Chemistry Mock Test - 4
The First Cotton Textile Mill was established in India at
Profit in a transaction is 80%. If the cost increases by 20%, then what is the change in profit percentage, if the selling price is the same?
If '+' means '×', '×' means '–', and '÷' means '+', then which of the following would be the value of 14 ÷ 2 + 8 × 5?
Web search engines works with the help of two programs. Which are they?
What will be the position of the cursor when the HOME command is given?
The academic performance of students can be improved if parents are encouraged to
Only One Option Correct Type
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q. Which type of isomerism is shown by [Co(NH3)4 Br2CI]?
Which of the following complex will give white precipitate with barium chloride solution?
when this equation is balanced, the num ber of OF- ions added
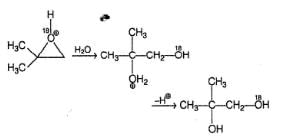
In the following hydrolysis reactio, the major final product is
The reaction of cyanamide, NH2CN (s) was carried out in a bomb calorimeter, and ΔU was found to be - 742.7 kJ mol-1 at 298 K. Thus, ΔrH° at 298 K for
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct
An ethanol-water solution is prepared by dissolving 10.00 mL o f ethanol (C2H5OH, d = 0.789 g mL-1) in a sufficient volume o f w ater to produce 100 mL of solution with a density, d = 0.982 g mL-1. Match the concentration term in Column I with its value in Column II and select the answer from the code
In which of the following complexes the nickel metal is in highest oxidation state.
Mean bond enthalpy of different bonds are given
Out of the given pairs, which compound is more stable than the other?
Passage II
An organic compound X (C7H11Br) shows optical isomerism as well as decolourises brown colour of bromine water solution. X on treatment with HBr in the absence of a peroxide forms a pair of diastereomers, both of them are optically active. Also, X with C2H5ONa in C2H5OH gives a single product Y (C7H10). Y on treatment with ozone followed by reduction with (CH3)2S gives 1, 3,-cyclopentanedione as one product.
Q. The correct statement concerning product(s) formed when Y is treated with excess of HCI is
Sum of atomic mass of all atoms present in one molecule of a molecular compound is
Following mechanism has been proposed for a reaction
The rate law expression for the reaction is
Determinants of individual differences in human beings relate to