Test: Chemistry - 1 - UPSC MCQ
20 Questions MCQ Test Science & Technology for UPSC CSE - Test: Chemistry - 1
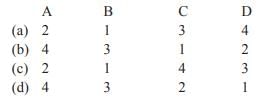
Match List-I with List-II and select the correct answer from the codes given below:




Match List-I with List-II and select the correct answer given below:



| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Consider the following parts of spectra:
1. Visible
2. Infrared
3. Ultraviolet
4. Microwave
Q. Which of the following is the correct sequence in which the wavelengths increase?
Match List-I with List-II and select the correct answer from the codes given below:


The difference between a nuclear reactor and atomic bomb is that
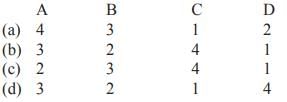
Match List-I with List-II and select the correct answer from the codes given below

Consider the following statements and select the correct code.
Assertion (A): A chemical reaction becomes faster at higher temperature.
Reason (R): At higher temperature, molecular motion becomes more rapid.
The order of appearance of the following with increasing temperature during the refining of crude oil is __________
Consider the following statements: If there were no phenomenon of capillarity
1. It would be difficult to use a kerosene lamp.
2. One would not be able to use a straw to consume a soft drink.
3. the blotting paper would fail to function.
4. the big trees that we see around would not have grown on the earth.
Q. Which of the statements given above is/are correct?
The blue colour of water in the sea. What is the reason behind the phenomenon?
Q.Which of the following substances is/are ozone depleting?
1. Chlorofluorocarbons
2. Halons
3. Carbon tetrachloride
While tinning of brass utensils, the ammonium chloride powder used to clean the hot utensil produces fumes of
Hydrofluoric acid is not kept in glass bottles because it reacts with
|
146 videos|358 docs|249 tests
|
|
146 videos|358 docs|249 tests
|



















