Test: Properties of Hydrocarbons - Class 10 MCQ
15 Questions MCQ Test Science Class 10 - Test: Properties of Hydrocarbons
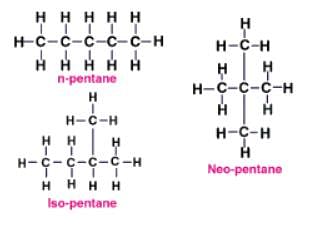
Which of the following is undergoing the substitution reaction?
The following chemical reaction shows the addition of chlorine to methane in the presence of sunlight:
CH4 + Cl2 → X
What is likely to be the product of the reaction represented by “X”?
CH4 + Cl2 → X
What is likely to be the product of the reaction represented by “X”?
The products obtained after the combustion of methane are:
Which of the following belongs to a homologous series of alkynes?
Which of the following hydrocarbon does not undergo addition reaction?
The oxidising agent used to convert alcohols into carboxylic acid is:
In the hydrogenation of vegetable oils, the unsaturated hydrocarbons generally add hydrogen in the presence of:
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that:
The reaction in which oxidising agents supply nascent oxygen for oxidation of alcohols to their respective acids is known as:
The conversion of butene to butane in presence of nickel is an example of:
|
83 videos|438 docs|74 tests
|