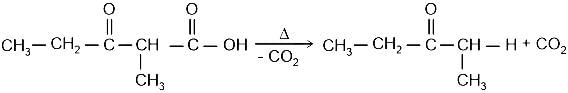CUET PG Chemistry Mock Test - 2 - CUET PG MCQ
30 Questions MCQ Test Chemistry CUET PG Mock Test Series 2026 - CUET PG Chemistry Mock Test - 2
Given below are two statements:
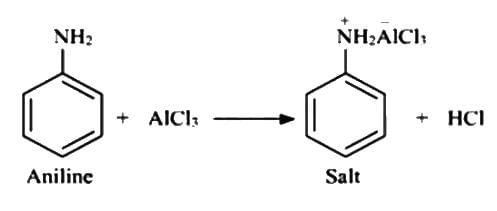
Statement (I) : The NH2 group in Aniline is ortho and para directing and a powerful activating group.
Statement (II) : Aniline does not undergo FriedelCraft’s reaction (alkylation and acylation).
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement (I) : The NH2 group in Aniline is ortho and para directing and a powerful activating group.
Statement (II) : Aniline does not undergo FriedelCraft’s reaction (alkylation and acylation).
In the light of the above statements, choose the most appropriate answer from the options given below :
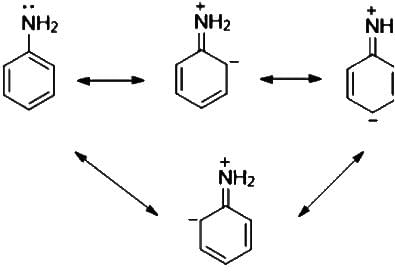
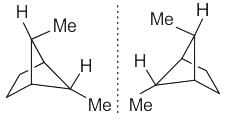
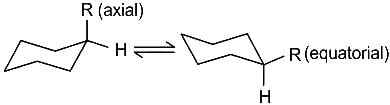
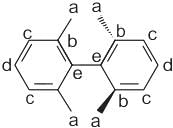
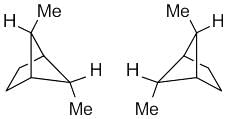
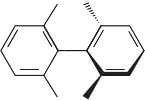
The correct relationship between the following structures is that they are
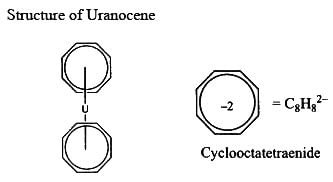
For uranocene, the correct statement(s) is/are:
(A) oxidation state of uranium is '+4'.
(B) it has cyclooctatetraenide ligands
(C) it is a bent sandwich compound
(D) it has '-2 ' charge.
Correct answer is
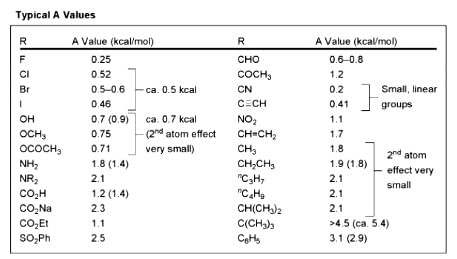
The correct order of magnitude of ‘A values’ for the given substituents in cyclohexane derivatives is
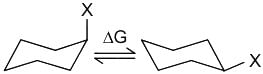
X = Me
X = F
X = Br
X = tBu
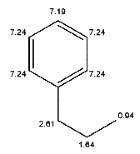
The structure of the compound having molecular formula C9H12 showing NMR peaks at δ 7.1, 2.2, 1.5 and 0.9 ppm is
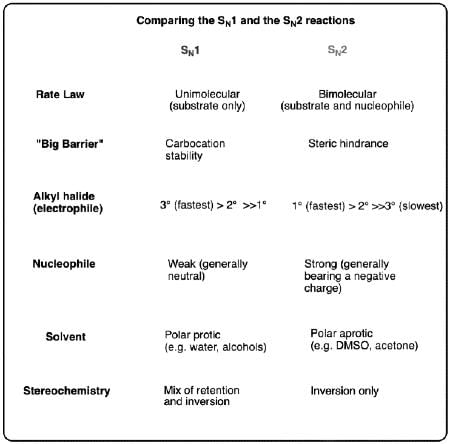
In S1N and S2N reactions
(A). S1N is a unimolecular reaction with first order kinetics while S2N reaction is bimolecular reaction with second order kinetics.
(B). In S2N, the reaction proceeds through the formation of carbocation while S1N does not.
(C). S2N is a stereospecific reaction. The product formed with inversion of configuration only.
(D). S1N reaction is favoured in the presence of weak base or poor nucleophile.
Choose the correct answer from the options given below:
The co-ordination number of Gd in GdCl3.6H2O is -
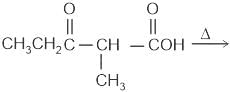
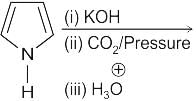
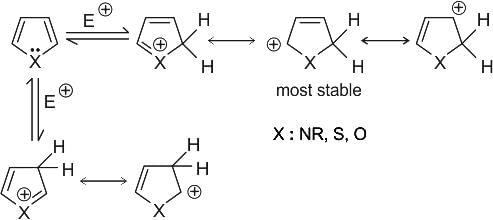
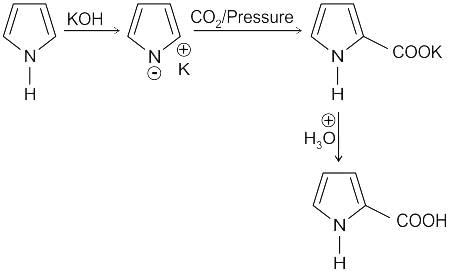
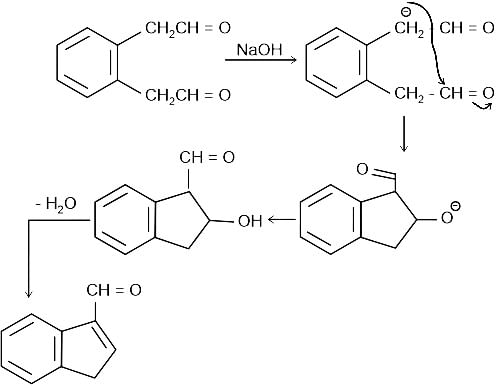
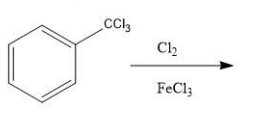
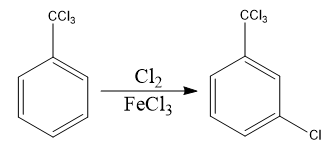
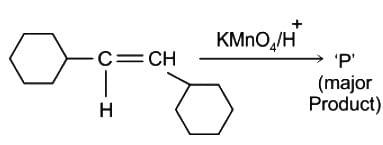
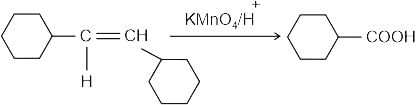
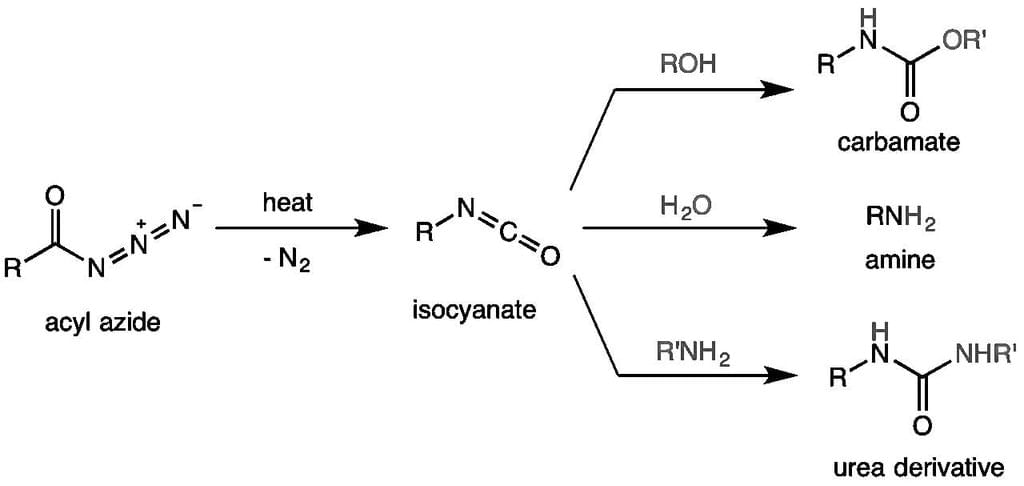
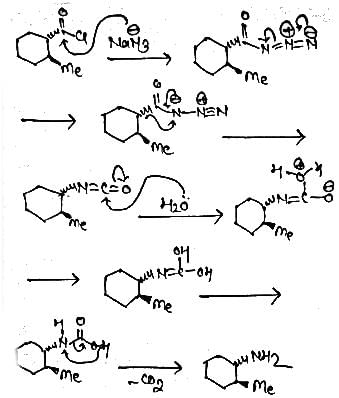
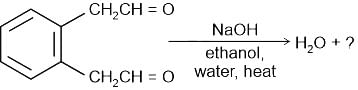
Identify the principal product of the following reaction?
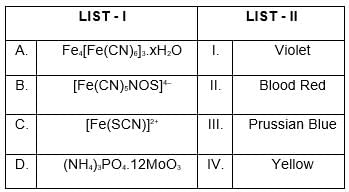
Match List I with List II
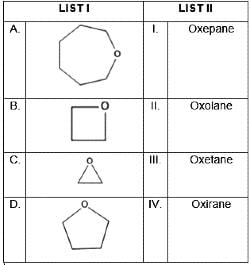
Choose the correct answer from the options given below:
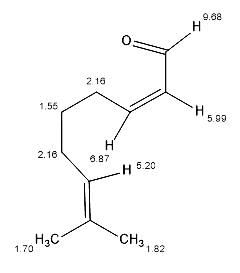
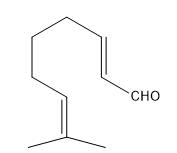
Find the number of 1H signals in the following compound.
Magnetic moment of a complex is 2.83 BM. The complex ion is
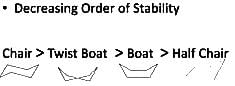
The correct order of the stability of different conformations of cyclohexane is
(A). chair
(B). boat
(C). twist boat
D). half chair
Arrange the following compounds A,B,C and D in decreasing order of acidic strength
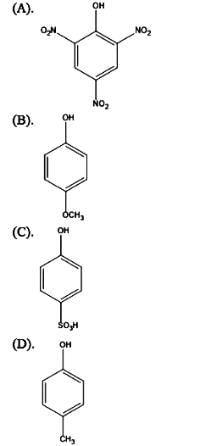
Choose the correct answer from the options given below:
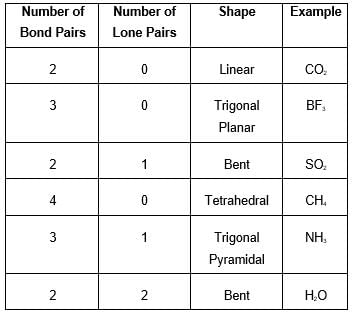
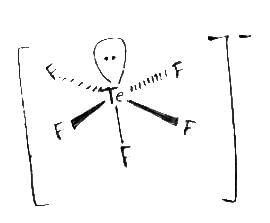
The correct shape of [TeF5]– ion on the basis of VSEPR theory is
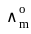 for Ba(OH)2 is
for Ba(OH)2 is
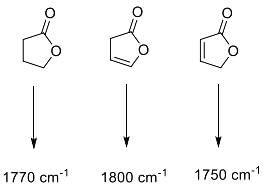
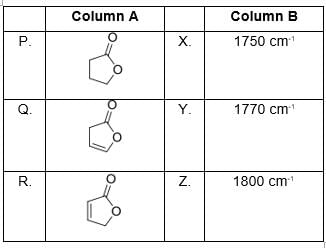
Correctly matched structure and carbonyl stretching frequency
Match List I with List II
Choose the correct answer from the options given below :
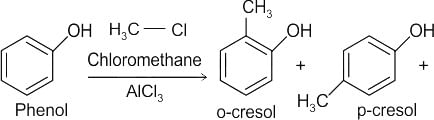
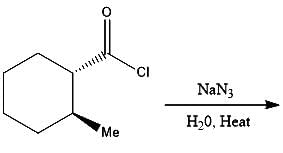
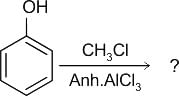
What will be the product of the following reaction?
Number of microstates available for a p3 configuration is -
The number of signals observed in the proton decoupled 13C NMR spectrum of the following compound is
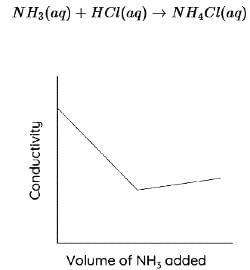
In the conductometric titration of hydrochloric acid against ammonium hydroxide
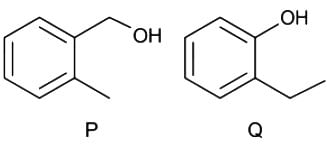
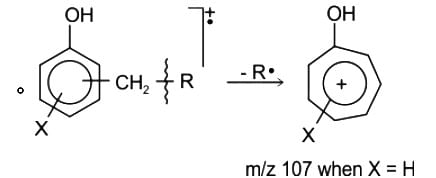
The base peaks (m/z) in the El mass spectra of compounds P and Q appear, respectively, at