Test: Chemistry and Metabolism of Amino Acids- 2 - NEET PG MCQ
25 Questions MCQ Test - Test: Chemistry and Metabolism of Amino Acids- 2
Flexibility of protein depends on: (AI 1994)
Which amino acid can protonate and deprotonate at neutral pH? (AIIMS May 95)
Phosphorylation of amino acid by: (PGI June 98)
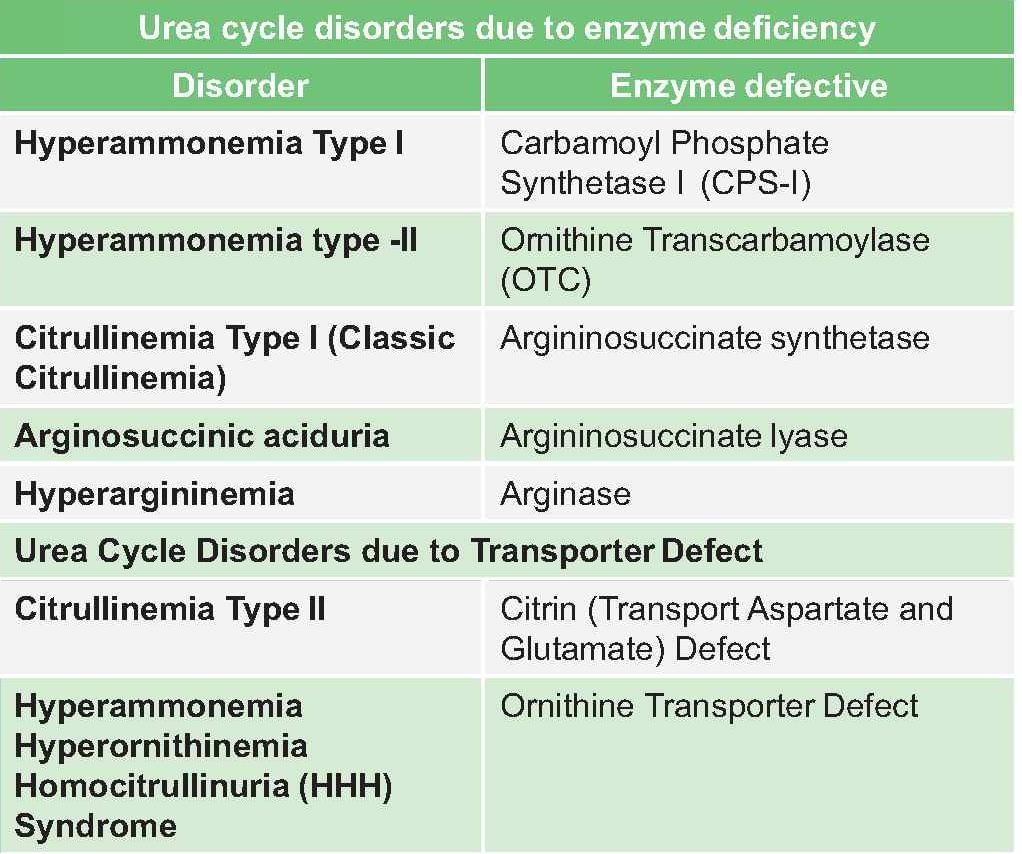
Which enzymes are part of urea cycle?
In which of the following condition there is increased level of ammonia in blood? (Ker 2008)
Glutamate dehydrogenase in mitochondria is activated by:
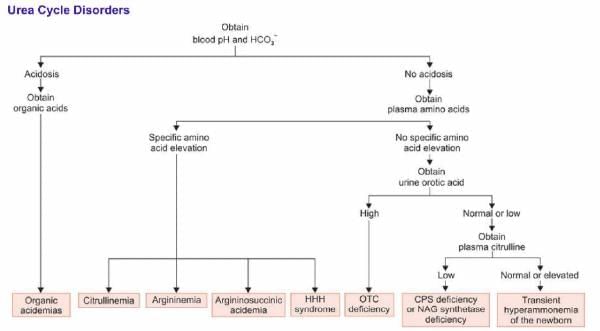
A 6-month-old boy admitted with failure to thrive with high glutamine and Uracil in urine Hypoglycemia, high blood ammonia. Treatment given for 2 months. At 8 months again admitted for failure to gain weight. Gastric tube feeding was not tolerated Child became comatose. Parenteral Dextrose given. Child recovered from coma within 24 hours. What is the enzyme defect?
Which of the following is true in relation of urea cycle? (PGI Dec 05)
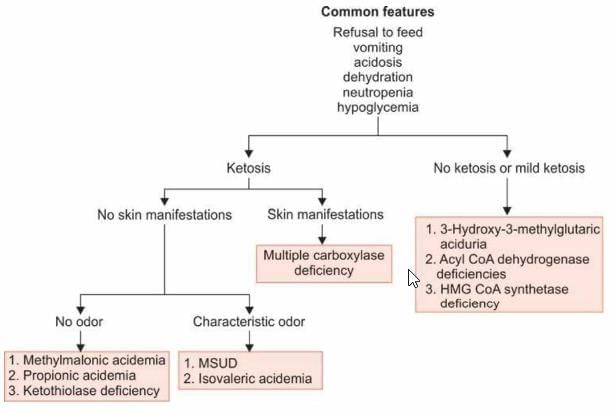
A baby presents with refusal to feed, skin lesions, seizures, ketosis, organic acids in urine with normal ammonia; likely diagnosis: (AI 2001)
All are true regarding urea cycle except:
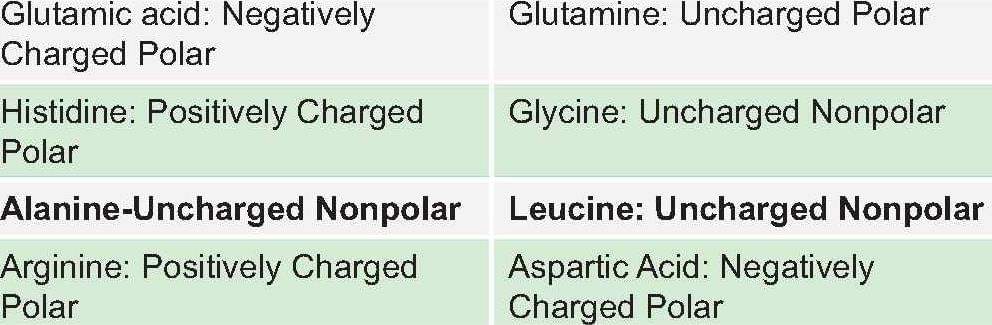
Which of these is a conservative mutation: