NEET PG Exam > NEET PG Tests > Test: Proteins- 2 - NEET PG MCQ
Test: Proteins- 2 - NEET PG MCQ
Test Description
25 Questions MCQ Test - Test: Proteins- 2
Test: Proteins- 2 for NEET PG 2025 is part of NEET PG preparation. The Test: Proteins- 2 questions and answers have been prepared
according to the NEET PG exam syllabus.The Test: Proteins- 2 MCQs are made for NEET PG 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Proteins- 2 below.
Solutions of Test: Proteins- 2 questions in English are available as part of our course for NEET PG & Test: Proteins- 2 solutions in
Hindi for NEET PG course.
Download more important topics, notes, lectures and mock test series for NEET PG Exam by signing up for free. Attempt Test: Proteins- 2 | 25 questions in 25 minutes | Mock test for NEET PG preparation | Free important questions MCQ to study for NEET PG Exam | Download free PDF with solutions
Detailed Solution for Test: Proteins- 2 - Question 1
Test: Proteins- 2 - Question 2
All of the following are true about Sickle cell disease, except: (AI 2008)
Detailed Solution for Test: Proteins- 2 - Question 2
Detailed Solution for Test: Proteins- 2 - Question 3
Test: Proteins- 2 - Question 4
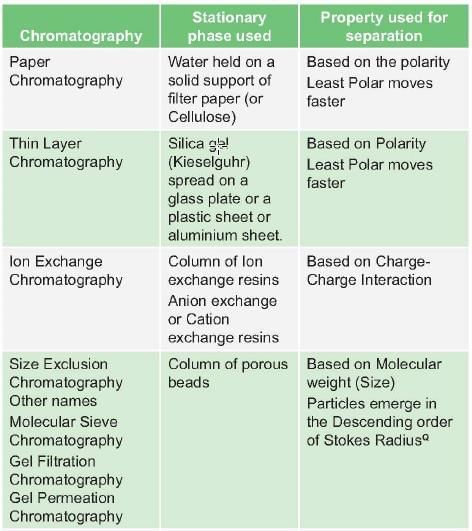
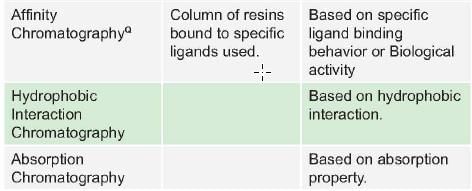
Protein separation based on mass/mol wt (size) is/ are done in all except: (PGI May 2012)
Detailed Solution for Test: Proteins- 2 - Question 4
*Multiple options can be correct
*Multiple options can be correct
Test: Proteins- 2 - Question 6
In SDS-PAGE, proteins are separated on basis of: (PGI June 2009)
Detailed Solution for Test: Proteins- 2 - Question 6
Test: Proteins- 2 - Question 7
Method of chromatography in which molecules that are negatively charged are selectively released from stationary phase into the positively charged molecules in mobile phase is termed as: (AI 2010)
Detailed Solution for Test: Proteins- 2 - Question 7
Test: Proteins- 2 - Question 8
Movement of protein from nucleus to cytoplasm can be seen by? (AIIMS 2010)
Detailed Solution for Test: Proteins- 2 - Question 8
Detailed Solution for Test: Proteins- 2 - Question 9
Detailed Solution for Test: Proteins- 2 - Question 10
*Multiple options can be correct
Detailed Solution for Test: Proteins- 2 - Question 11
Test: Proteins- 2 - Question 12
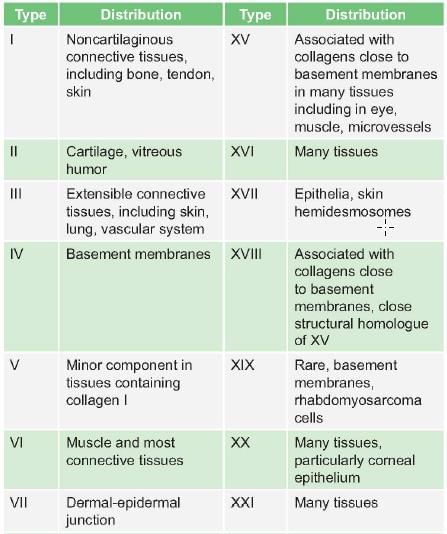
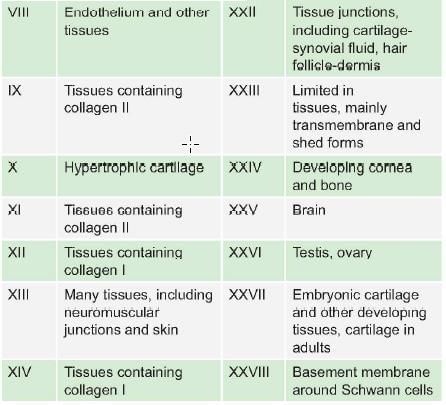
Collagen of which type is found in hyaline cartilage: (AIIMS Nov 2007)
Detailed Solution for Test: Proteins- 2 - Question 12
*Multiple options can be correct
Detailed Solution for Test: Proteins- 2 - Question 13
Test: Proteins- 2 - Question 14
Keratin is present in both skin and nail. But nail is harder than skin. The reason is: (AI 2012)
Detailed Solution for Test: Proteins- 2 - Question 14
Test: Proteins- 2 - Question 15
The structural proteins are involved in maintaining the shape of a cell or in the formation of matrices in the body. The shape of these protein is: (AI 2006)
Detailed Solution for Test: Proteins- 2 - Question 15
Detailed Solution for Test: Proteins- 2 - Question 16
Test: Proteins- 2 - Question 17
All of the following are required for hydroxylation of proline in collagen synthesis except: (Al 1997)
Detailed Solution for Test: Proteins- 2 - Question 17
Detailed Solution for Test: Proteins- 2 - Question 18
Detailed Solution for Test: Proteins- 2 - Question 19
Detailed Solution for Test: Proteins- 2 - Question 20
Detailed Solution for Test: Proteins- 2 - Question 21
Detailed Solution for Test: Proteins- 2 - Question 22
Detailed Solution for Test: Proteins- 2 - Question 23
Test: Proteins- 2 - Question 24
Targeting sequence that direct Endoplasmic Reticulum resident protein in retrograde flow to ER in COP-I vesicles.
Detailed Solution for Test: Proteins- 2 - Question 24
Detailed Solution for Test: Proteins- 2 - Question 25
Information about Test: Proteins- 2 Page
In this test you can find the Exam questions for Test: Proteins- 2 solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Proteins- 2, EduRev gives you an ample number of Online tests for practice
Download as PDF