VITEEE Chemistry Test - 10 - JEE MCQ
30 Questions MCQ Test - VITEEE Chemistry Test - 10
Ethylene may be obtained by the treatment of concentrated H₂SO₄ and X at 160-1700C. X is
Aromatic aldehydes undergo disproportionation in presence of sodium or potassium hydroxide to give corresponding alcohol and acid. The reaction is known as
A carbonyl compound reacts with hydrogen cyanide to form cyanohydrin which on hydrolysis forms a racemic mixture of α-hydroxy acid. The carbonyl compound is
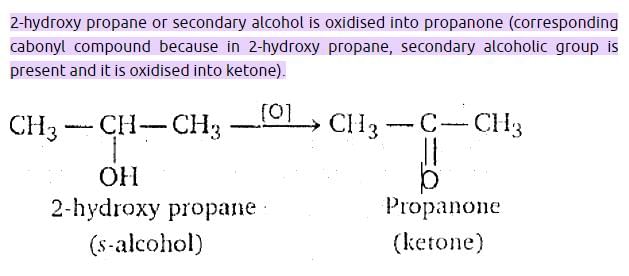
Which one of the following can be oxidised to the corresponding carbonyl compounds?
Which alkene gives the same product by the Markownikoff's and anti-Markownikoff's method
Wurtz reaction involves the interaction of alkyl halides in dry ether with
Among the following pairs, the one which illustrates the law of multiple proportions is
When propanamide reacts with Br2 and NaOH then which of the following compounds is formed ?
A carboxylic acid is converted into its anhydride using
A liquid is in equilibrium with its vapour at its boiling point. The molecules in these two phases will have equal
The rate of forward reaction is two times that of the reverse reaction at a given temperature and identical concentration, K equilibrium is
A Carnot engine, whose efficiency is 20% receives heat at 450 K. If its efficiency is increased to 30%, then the intake temperature for the same exhaust temperature is
The enthalpy of a solution of KNO3 is + 35.64 kJ. This denotes
Which type of isomerism,is shown by 2,3-dichloro butane ?
Prussian blue is obtained by mixing together aqueous solution of Fe3+ salt with
Which of the following is produced by reaction of RCN in sodium and alcohol ?
The equivalent conductivity of 0.1 M weak acid is 100 times less than that at infinite dilution. The degree of dissociation is
When ether is exposed in air for sometime an explosive substance produced is
Which of the following method is used for the purification of titanium?
The volume of 2.8 g of CO at 270C and 0.821 atm. pressure is (R=0.0821 lit. atm mol-1K-1)
Method used for obtaining highly pure silicon used as a semiconductor material, is