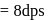Test: Rate of reactions - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Rate of reactions
The reaction rate is defined as the rate at which the concentration of the reactants __________ with time or the concentration of products ___________ with time.
Consider the following reaction
A⟶ Products
This reaction is completed in 100 min. The rate constant of this reaction at t1 = 10 min, is 10−2 min−1. What is the rate constant (in min−1) at t2 = 20 min?
A⟶ Products
This reaction is completed in 100 min. The rate constant of this reaction at t1 = 10 min, is 10−2 min−1. What is the rate constant (in min−1) at t2 = 20 min?
Which of the following terms would not be determined experimentally?
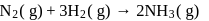
The equality relationship between
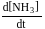 and
and 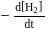 is
is
A following mechanism has been proposed for reaction 2 A + B → D + E
A + B → C + D(slow)
A + C → E (fast)
The rate law expression for the reaction is:
The rate law for the reaction 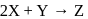 is Rate
is Rate 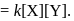 The correct statement with regard to this relation is
The correct statement with regard to this relation is
In a reaction, 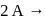 products, the concentration of
products, the concentration of 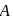 decreases from
decreases from 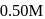 to
to 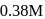 in
in 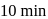 . What is the rate of disappearance of A (in
. What is the rate of disappearance of A (in 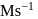 ) during this interval?
) during this interval?
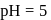 for any concentration of sugar. However if
for any concentration of sugar. However if 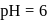 , the half-life changes to 50 minute. The rate law expression for the sugar inversion can be written as
, the half-life changes to 50 minute. The rate law expression for the sugar inversion can be written as
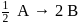 , rate of disappearance of '
, rate of disappearance of ' 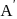 is related to the rate of appearance of 'B' by the expression
is related to the rate of appearance of 'B' by the expression
For the reaction 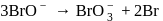 in alkaline aqueous solution, the value of the second order (in
in alkaline aqueous solution, the value of the second order (in 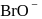 ) rate constant at
) rate constant at 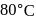 in the rate law
in the rate law 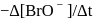 was found to be
was found to be
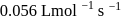 . Rate constant when the rate law is written for
. Rate constant when the rate law is written for 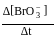 is
is
A G.M. counter is used to study the radioactive process of first-order. In the absence of radioactive substance 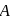 , it counts 3 disintegration per second (dps). When
, it counts 3 disintegration per second (dps). When 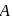 is placed in the G.M. counter, it records 23 dps at the start and 13 dps after 10 minutes. It records
is placed in the G.M. counter, it records 23 dps at the start and 13 dps after 10 minutes. It records 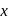 dps after next 10 minutes and
dps after next 10 minutes and 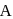 has half-life period y minutes.
has half-life period y minutes. 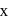 and
and 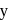 are
are
|
361 videos|822 docs|301 tests
|


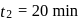 also
also 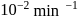 (constant).
(constant).
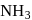 and
and 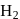 , then
, then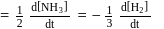
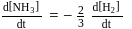
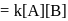

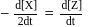
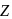 is half of the rate of disappearance of
is half of the rate of disappearance of 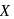 .
.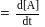
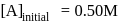
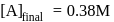
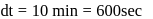
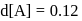
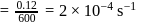 .
.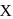 Hence X
Hence X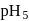 , the half-life is independent of the concentration of sugar. Hence, the reaction is of first order in sugar.
, the half-life is independent of the concentration of sugar. Hence, the reaction is of first order in sugar.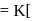 sugar
sugar 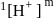 .
.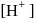 is
is 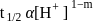
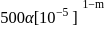
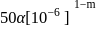
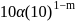
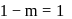
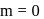
 .
.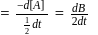 or
or 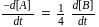
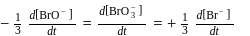
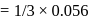
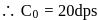
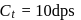
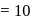 minutes
minutes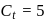 dps
dps