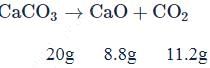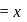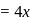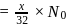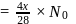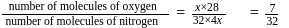Test: Mole concept - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Mole concept
1.44 g of titanium (At. mass = 48) reacted with excess of O2 and produce 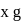 of non stoichiometric compound
of non stoichiometric compound 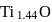 . The value of
. The value of 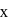 is:
is:
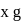 of non stoichiometric compound
of non stoichiometric compound 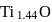 . The value of
. The value of 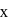 is:
is:The mass of 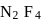 produced by the reaction of
produced by the reaction of 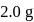 of
of 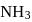 and
and 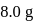 of
of 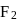 is
is 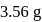 . What is the per cent yield?
. What is the per cent yield?
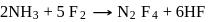
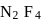 produced by the reaction of
produced by the reaction of 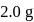 of
of 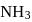 and
and 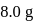 of
of 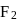 is
is 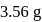 . What is the per cent yield?
. What is the per cent yield?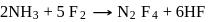
The impure 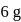 of
of 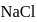 is dissolved in water and then treated with excess of silver nitrate solution. The mass of precipitate of silver chloride is found to be
is dissolved in water and then treated with excess of silver nitrate solution. The mass of precipitate of silver chloride is found to be 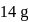 . The
. The 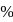 purity of
purity of 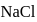 solution would be:
solution would be:
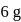 of
of 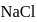 is dissolved in water and then treated with excess of silver nitrate solution. The mass of precipitate of silver chloride is found to be
is dissolved in water and then treated with excess of silver nitrate solution. The mass of precipitate of silver chloride is found to be 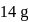 . The
. The 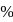 purity of
purity of 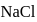 solution would be:
solution would be:What is the empirical formula of vanadium oxide, if 2.74 g of the metal oxide contains 1.53 g of metal?
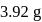 of ferrous ammonium sulphate crystals are dissolved in
of ferrous ammonium sulphate crystals are dissolved in 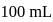 of water.
of water. 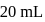 of this solution requires
of this solution requires 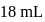 of potassium permaganate during titration for complete oxidation. The weight of
of potassium permaganate during titration for complete oxidation. The weight of 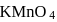 present in one litre of the solution of
present in one litre of the solution of
Specific volume of cylindrical virus particle is 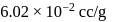 . whose radius and length are
. whose radius and length are 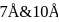 respectively.
respectively.
If 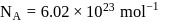 , find molecular weight of virus
, find molecular weight of virus
10 moles 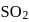 and 15 moles
and 15 moles 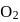 were allowed to react over a suitable catalyst. 8 moles of
were allowed to react over a suitable catalyst. 8 moles of 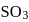 were formed. The remaining moles of
were formed. The remaining moles of 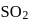 and
and 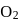 respectively are -
respectively are -
On reduction with hydrogen 3.6 g of an oxide of metal leaves 3.2 g of metallic residue. If the atomic mass of metal is 64 , the formula of metal oxide is
Vapour density of a metal chloride is 83. If equivalent weight of the metal is 6, its atomic weight will be ____.
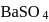 formed upon mixing
formed upon mixing 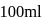 of
of 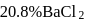 solution with 50
solution with 50 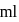 of
of 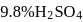 solution will be:
solution will be:(Given that molecular weight of
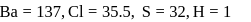 and
and 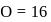

9 moles of "D" and 14 moles of E are allowed to react in a closed vessel according to given reactions. Calculate number of moles of B formed in the end of reaction, if 4 moles of G are present in reaction vessel. (Percentage yield of reaction is mentioned in the reaction)
Step-1 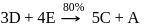
Step-2 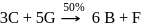
A mixture of 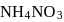 and
and 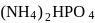 contain
contain 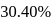 mass per cent of nitrogen. What is the mass ratio of the two components in the mixture?
mass per cent of nitrogen. What is the mass ratio of the two components in the mixture?
A gaseous mixture contains oxygen and nitrogen in the ratio of 1: 4 by weight. Therefore, the ratio of their number of molecules is
|
361 videos|822 docs|301 tests
|


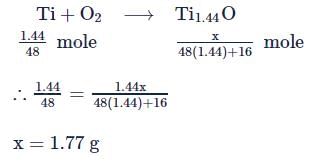
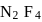 formed by
formed by 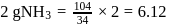
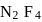 formed by
formed by 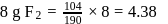
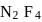 will be limiting and actual amount of product is
will be limiting and actual amount of product is 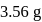
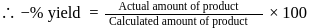
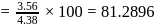

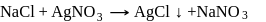
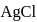 is produced from
is produced from 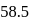
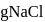
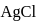 will be produced from
will be produced from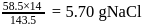
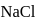 in common salt:
in common salt: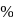 purity
purity 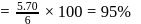
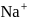 ions are in 20 ml of 0.4 M
ions are in 20 ml of 0.4 M 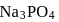 ?
?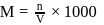
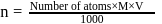
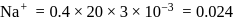
 Mass of metal
Mass of metal  Mass of oxygen
Mass of oxygen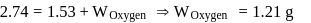
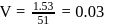
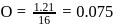
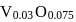
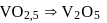
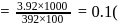 Eq. wt of FAS is 392
Eq. wt of FAS is 392
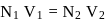
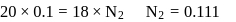
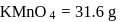
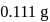 ev. of
ev. of 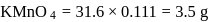 .
.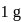 ) of cylindrical virus particle
) of cylindrical virus particle 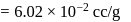
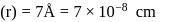
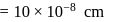
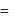
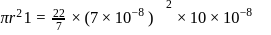
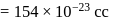
 Wt. of
Wt. of 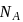 particle
particle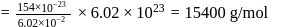
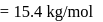
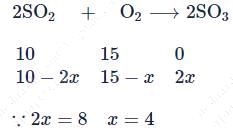
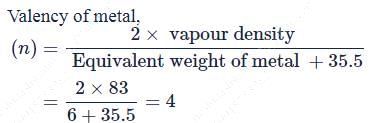
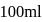 of
of 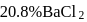 solution
solution 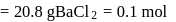 .
.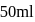 of
of 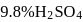 solution
solution 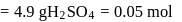 .
.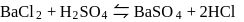
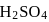 is the limiting reagent, only
is the limiting reagent, only 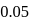 moles of
moles of 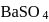 will form.
will form.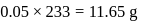 of
of 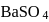 is formed.
is formed.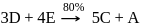
 mole
mole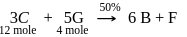
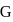
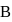 formed
formed 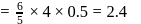
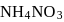 and
and 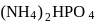 are
are 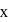 and y gram respectively
and y gram respectively 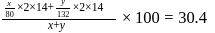
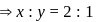
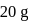 of
of 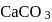 on heating gave
on heating gave 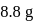 of
of 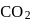 and
and 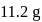 of
of 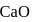 . This is in accordance with
. This is in accordance with