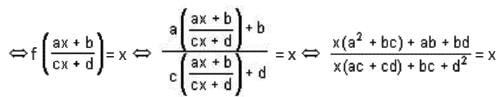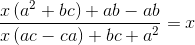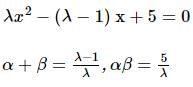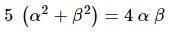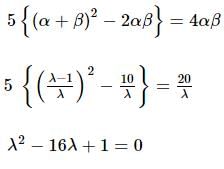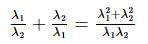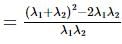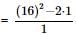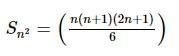SRMJEE Mock Test - 2 (Engineering) - JEE MCQ
30 Questions MCQ Test - SRMJEE Mock Test - 2 (Engineering)
Monochromatic light is refracted from air into glass of refractive index μ. The ratio of the wavelengths of the incident and refracted waves is
A proton moving with a speed u along the positive x-axis enters at y = 0, a region of uniform magnetic field B = B0 which exists to the right of y-axis as shown in the figure. The proton leaves the region after some time with a speed v at coordinate y. Then,
which exists to the right of y-axis as shown in the figure. The proton leaves the region after some time with a speed v at coordinate y. Then,

The radius of an air bubble at the bottom of a lake is r and it becomes 2r when the air bubble rises to the surface of the lake. If P is the atmospheric pressure of water, then the depth (in cm) of the lake is
In Young’s double-slit experiment, the separation between the slits is halved and the distance between the slits and the screen is doubled. The fringe width will
Assertion: If a varying current is flowing through a machine of iron, eddy currents are produced
Reason: Change in the magnetic flux through an area causes eddy currents.
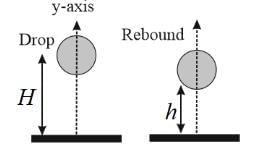
A ball of mass 50 g is dropped from a height H = 10 m. It rebounds, losing 75% of its kinetic energy. If it remains in contact with the ground for Δt = 0.01 s, the impulse of the impact force is:
A metal cube of length 10.0 mm at 0°C (273 K) is heated to 200°C (473 K). Given: its coefficient of linear expansion is 2 × 10⁻⁵ K⁻¹. The percent change in its volume is:
An amine (X) reacts with benzenesulphonyl chloride and the product thus obtained is soluble in KOH.
The amine (X) is
Reaction of phenol with NaOH followed by heating with CO2 under high pressure, and subsequent acidification gives compounds X as the major product, which can be purified by steam distillation. When reacted with acetic anhydride in the presence of a trace amount of conc. H2SO4 compound, X produces Y as the major product.
Compound Y is

During Hoffmann ammonlysis reaction, which of the following cannot be used for the formation of amines?
Followed by reaction with an excess of mercuric chloride, on heating with CS2 primary amines, yield isothiocyanates. What is the reaction called?
Among the second period elements, the actual first ionisation enthalpy are in order of? (Select the correct option from the following.)
If the function f : [1, ∞) → [1, ∞) is defined by f(x) = 2x(x - 1), then f-1 (x) is
Out of 6 students in a group, 3 come from first year, 2 come from second year and 1 comes from third year. In how many ways can they be arranged in a queue so that the same year students stand together?
The ratio of the circumradius and inradius of an equilateral triangle is:
If α, β are the roots of the equation λ(x² − x) + x + 5 = 0. If λ₁ & λ₂ are two values of λ for which the roots α, β are related by α/β + β/α = 4/5, then the value of λ₁/λ₂ + λ₂/λ₁ must be equal to:
If sin⁻¹(x) + sin⁻¹(y) = 2π/3, then find the value of cos⁻¹(x) + cos⁻¹(y).
Abhijit purchased a TV set for ₹18000 and a DVD player for ₹4000. He sold both the items together for ₹26400
₹26400. How much percent profit did he make?
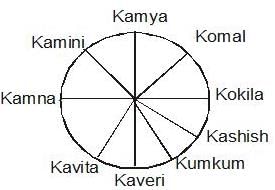
Study the following information carefully and answer the question given below.
Kamini, Komal, Kavita, Kamya, Kashish, Kumkum, Kaveri, Kokila and Kamna are sitting at a round table, facing the center. Komal is fourth to the right of Kaveri and second to the left of Kamini. Kashish is fourth to the left of Kamini and third to the right of Kavita. Kumkum is third to the right of Kamna. Kokila is second to the left of Kamya.
Who is second to the right of Kamini?
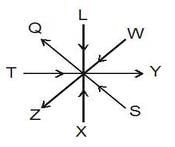
Read the following information carefully and answer the questions given below
Eight members X, Y, Z, S, L, Q, T and W are sitting around a circle. Three persons are facing out side and five persons are facing inside. Y is sitting second to the right of X and third to the left of Z. Q is not the neighbour or X and Y. W is sitting second to the right of Q who is second to the right of Z. L is second to the left of Y and third to the right of S.
How many persons are sitting between L and Z, anti-clockwise with respect to Z?


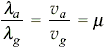
 B); where q is the charge of the proton. The force F is perpendicular to both u and B. Since the force is perpendicular to the velocity of the particle, it does not do any work. Hence, the magnitude of the velocity of the particle will remain unchanged; only the direction of the velocity changes. Hence, v = u. Since u is perpendicular to B, the proton moves in a circular path. Since the charge of proton is positive, u is along positive x-axis and B is directed out of the page; the proton will move in a circle in the x-y plane in the clockwise direction. Hence, its y-coordinate will be negative, when it leaves the region. Thus, the correct choice is (4).
B); where q is the charge of the proton. The force F is perpendicular to both u and B. Since the force is perpendicular to the velocity of the particle, it does not do any work. Hence, the magnitude of the velocity of the particle will remain unchanged; only the direction of the velocity changes. Hence, v = u. Since u is perpendicular to B, the proton moves in a circular path. Since the charge of proton is positive, u is along positive x-axis and B is directed out of the page; the proton will move in a circle in the x-y plane in the clockwise direction. Hence, its y-coordinate will be negative, when it leaves the region. Thus, the correct choice is (4).
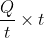 = Q. Hence, ampere-hour is the unit of electric charge.
= Q. Hence, ampere-hour is the unit of electric charge.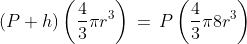
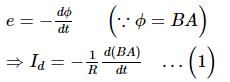





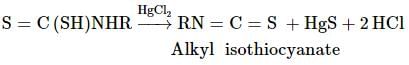
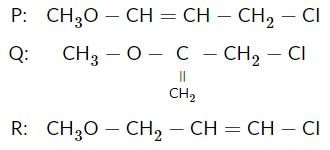

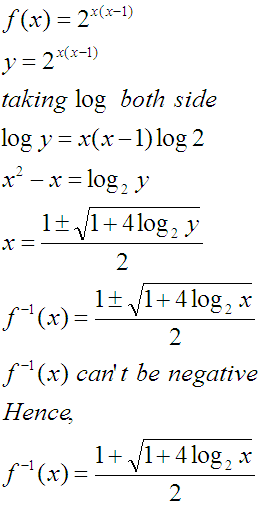


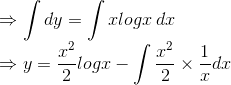

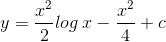
 .
.