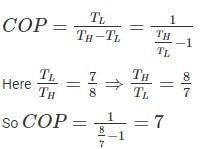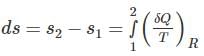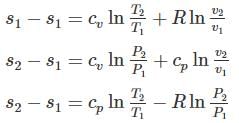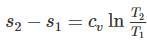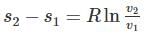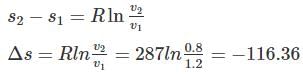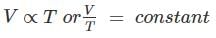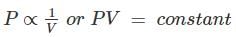Thermodynamics - 3 - Mechanical Engineering MCQ
20 Questions MCQ Test - Thermodynamics - 3
In a P-V diagram for pure substance, the constant temperature lines in saturated liquid-vapour region are ________.
If the entropy of the universe decreases. What this depicts about the nature of the process?
Which of the following properties are NOT sufficient to determine the properties of a vapour?
- Temperature
- Pressure
- Dryness fraction
- Specific volume
If the ratio of the lower to the higher absolute temperature is 7/8, then what will be the COP of the Carnot refrigerator?
One kg of air (R=287 J/kg-K) goes through an irreversible process between two equilibrium state 1 (30°C, 1.2 m3) and state 2 (30°C, 0.8 m3). What is the change in entropy (in J/kg-K)?
In a surrounding, the amount of irreversibility of a process undergone by a system is determined by ________.
Clausius’ statement and Kelvin - Planck’s statement are ________.
A Carnot engine operates between temperature 1000 K and 400 K. The heat rejected by the first Carnot engine is used by the second Carnot engine, whose sink temperature is 200 K. If the net heat absorbed by the first Carnot engine is 200 MJ. What is the heat rejected (in MJ) by the second Carnot engine?
In a general compression process, 2 kJ of mechanical work is supplied to 4 kg of fluid and 800 J of heat is rejected to the cooling jacket. The change in specific internal energy would be
Which of the below stated are properties of a PMM-2?
- When the net work is equal to the heat absorbed and work efficiency is 100%.
- Heat is exchanged from one heat reservoir only.
- It violates Kelvin-Planck statement.
- It is a hypothetical machine
A gas having a negative Joule-Thomson coefficient (μJ < 0), when throttled, will
According to which law, all perfect gases change in volume by (1/273)th of their original volume at 0°C for every 1°C change in temperature when pressure remains constant
For a single component three phase mixture, the number of independent variable properties are
Three states of matter are distinguished with respect to molecules by the ______.
An automobile heats up while lying in a parking lot on a sunny day. The process can be assumed to be
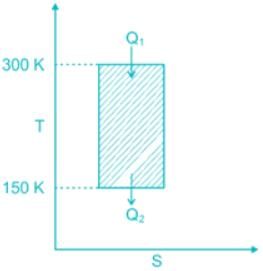
While working between temperatures 150 K and 300 K, the entropy change experienced by the Carnot engine during heat addition is 1 kJ/K, the work produced (kJ) by the engine is ________.