Test: Cannizzaro Reaction - NEET MCQ
23 Questions MCQ Test Chemistry Class 12 - Test: Cannizzaro Reaction
Only One Option Correct Type
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
The major organic product in the following reaction is
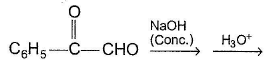
Which is the best hydride (H-) donor in the key step of Cannizaro reaction?
Consider the following reaction,
Q.
The major organic product is
What is the major organic product in the following reaction?
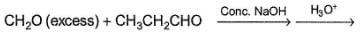
Predict the major organic product in the following reaction.
Whta is the major organic product formed in the follwing reaction ?
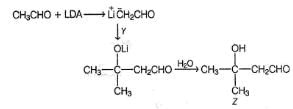
For a Cannizaro reaction,
Rate law is derived as : Rate = k [RCHO]2 [HO-]2
From the above rate law, it can be concluded that
Consider the following reaction,
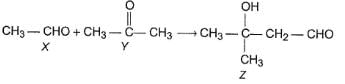
Q.
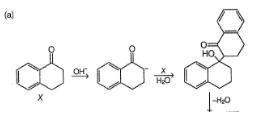
How the product Z can be prepared selectively using X and Y and other reagents?
What is the major product formed in the following intramolecular Cannizaro reaction?
One or More than One Options Correct Type
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Consider the cross Cannizaro reaction given below
Q.
The expected product(s) is/are
In the Cannizaro reaction mentioned below
Q.
The possible product(s) is/ar
What is/are the expected organic product(s) in the following reaction?
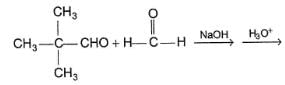
Consider the following reaction,
Q.
The expected organic product(s) is/are
Consider the following reaction sequence,
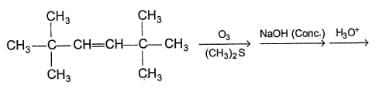
Q.
The expected organic product(s) is/are
Comprehension Type
Direction (Q. Nos. 16-18) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer mong the four given options (a), (b), (c) and (d).
Passage
When aldehydes lacking a-hydrogen is treated with concentrated solution of a strong base, Cannizaro reaction takes place. In this reaction, one molecule of aldehyde is oxidised while other is reduced. The widely accepted mechanism is
Step II
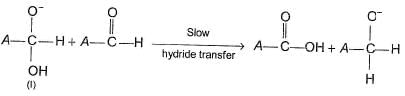
Q.
What is the rate law derived from the above mechanism?
When aldehydes lacking a-hydrogen is treated with concentrated solution of a strong base, Cannizaro reaction takes place. In this reaction, one molecule of aldehyde is oxidised while other is reduced. The widely accepted mechanism is
Step II
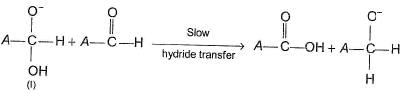
Q.
Which of the following observed fact establishes the correctness of the above mechanism?
When aldehydes lacking a-hydrogen is treated with concentrated solution of a strong base, Cannizaro reaction takes place. In this reaction, one molecule of aldehyde is oxidised while other is reduced. The widely accepted mechanism is
Step II

Q.
In certain experim ental condition, rate is found to be proportional to square of concentration of base (OH-).This indicates that
Matching List Type
Direction (Q. Nos. 19 and 20) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
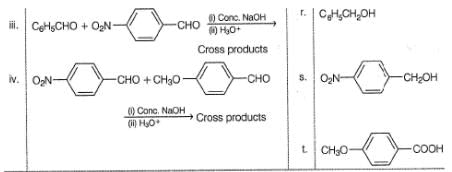
Consider the reactions of Column I and match with the products of Column II. Mark the correct option from the codes given below.

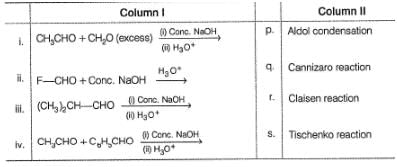
Match the reactions of Column I with the type of reactions from Column II. Mark the correct option from the codes given below.
One Integer Value Correct Type
Direction (Q. Nos. 21-23) This section contains 3 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Consider the following Cannizaro reaction,
Q.
How many different products are formed?
Consider the following Cannizaro reaction,
Q.
How many different products are expected in this reaction?
Consider the following reaction,
Q.
If CH3OH is formed exclusively by hydride transfer from a dianion intermediat what is the overall order of reaction?
|
75 videos|339 docs|78 tests
|