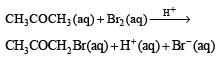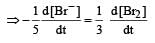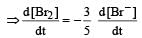31 Year NEET Previous Year Questions: Chemical Kinetics - NEET MCQ
30 Questions MCQ Test Chemistry Class 12 - 31 Year NEET Previous Year Questions: Chemical Kinetics
Half life period of a first-order reaction is 1386 seconds. The specific rate constant of the reaction is: [2009]
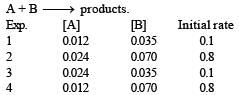
For the reaction A + B → products, it is observed that: [2009]
(1) On doubling the initial concentration of A only, the rate of reaction is also doubled and
(2) On doubling the initial concentrations of both A and B, there is a change by a factor of 8 in the rate of the reaction.
The rate of this reaction is given by:
(1) On doubling the initial concentration of A only, the rate of reaction is also doubled and
(2) On doubling the initial concentrations of both A and B, there is a change by a factor of 8 in the rate of the reaction.
The rate of this reaction is given by:
For the reaction 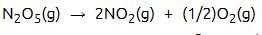 the value of rate of disappearance of N2O5 is given as 6.25 × 10–3 mol L–1s–1. The rate of formation of NO2 and O2 is given respectively as : [2010]
the value of rate of disappearance of N2O5 is given as 6.25 × 10–3 mol L–1s–1. The rate of formation of NO2 and O2 is given respectively as : [2010]
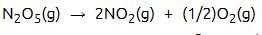 the value of rate of disappearance of N2O5 is given as 6.25 × 10–3 mol L–1s–1. The rate of formation of NO2 and O2 is given respectively as : [2010]
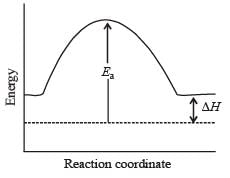
the value of rate of disappearance of N2O5 is given as 6.25 × 10–3 mol L–1s–1. The rate of formation of NO2 and O2 is given respectively as : [2010]For an endothermic reaction, energy of activation is Ea and enthalpy of reaction of ΔH (both of these in kJ/mol). Minimum value of Ea will be. [2010]
During the kinetic study of the reaction, 2A + B → C + D, following results were obtained:
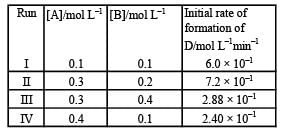
Based on the above data which one of the following is correct? [2010]
The rate of the reaction 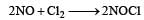 is given by the rate equation rate = k [NO]2 [Cl2] [2010] The value of the rate constant can be increased by:
is given by the rate equation rate = k [NO]2 [Cl2] [2010] The value of the rate constant can be increased by:
Which one of the following statements for the order of a reaction is incorrect ? [2011]
For the reaction 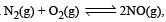 , the equilibrium constant is K1. The equilibrium constant is K2 for the reaction [2011]
, the equilibrium constant is K1. The equilibrium constant is K2 for the reaction [2011] 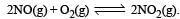 .What is K for the reaction
.What is K for the reaction 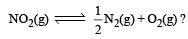
The rate of the reaction 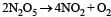 can be written in three ways :
can be written in three ways :
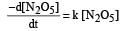
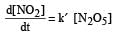
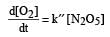
The relationship between k and k' and between k and k"are :
[2011 M]
The unit of rate constant for a zero order reaction is [2011 M]
In a reaction, A + B → Product, rate is doubled when the concentration of B is doubled, and rate increases by a factor of 8 when the concentrations of both the reactants (A and B) are doubled rate, law for the reaction can be written as : [2012]
In a zero-order reaction for every 10° rise of temperature, the rate is doubled. If the temperature is increased from 10°C to 100°C, the rate of the reaction will become : [2012]
Activation energy (Ea) and rate constants (k1 and k2) of a chemical reaction at two different temperatures (T1 and T2) are related by : [2012 M]
What is the activation energy for a reaction if its rate doubles when the temperature is raised from 20°C to 35°C? (R = 8.314 J mol–1 K–1) [NEET 2013]
A reaction having equal energies of activation for forward and reverse reaction has : [NEET 2013]
A reaction is 50% completed in 2 hours and 75% completed in 4 hours. The order of reaction is [NEET Kar. 2013]
For a reaction between A and B the order with respect to A is 2 and the order with respect to B is 3. The concentrations of both A and B are doubled, the rate will increase by a factor of: [NEET Kar. 2013]
Select the rate law that corresponds to data shown for the following reaction [1994]
For an exothermic reaction, the energy of activation of the reactants is [1994]
A chemical reaction is catalyzed by a catalyst X.Hence X [1995]
A substance 'A' decomposes by a first or der reaction starting initially with [A] = 2.00 m and after 200 min, [A] becomes 0.15 m. For this reaction t1/2 is [1995]
Activation energy of a chemical reaction can be determined by [1998]
Half life of a first order reaction is 4 s and the initial concentration of the reactants is 0.12 M.The concentration of the reactant left after 16 s is [1999]
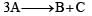 , it would be a zero order reaction when[2002]
, it would be a zero order reaction when[2002]
If the rate of the reaction is equal to the rate constant, the order of the reaction is [2003]
The reaction A → B follows first order kinetics.The time taken for 0.8 mole of A to produce 0.6 mole of B is 1 hour. What is the time taken for conversion of 0.9 mole of A to produce 0.675 mole of B?[2003]
For the reaction 2A + B → 3C + D which of the following does not express the reaction rate ? [2006]
If 60% of a first order reaction was completed in 60 minutes, 50% of the same reaction would be completed in aproximately [2007]
The bromination of acetone that occurs in acid solution is represented by this equation. [2008]
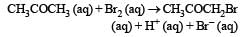
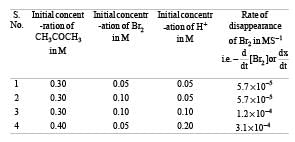
These kinetic data were obtained for given reaction concentrations.
Initial Concentrations, M
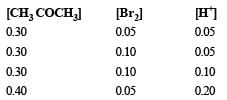
Initial rate, disappearance of Br2, Ms–1
5.7×10–5
5.7 × 10–5
1.2 × 10–4
3.1 × 10–4
Base on these data, the rate equations is:
In the reaction [2009]
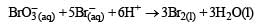
The rate of appearance of bromine (Br2) is related to rate of disappearance of bromide ions as following:
|
78 videos|351 docs|78 tests
|


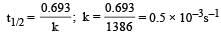

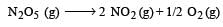
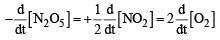
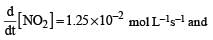
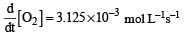
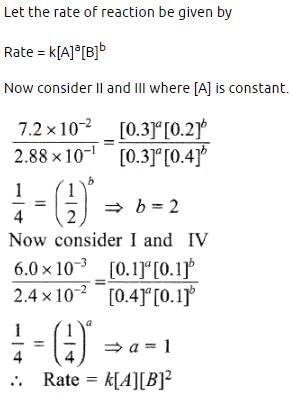
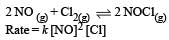
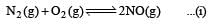
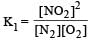
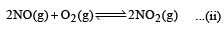
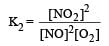
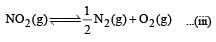
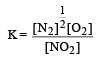
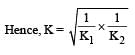
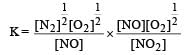
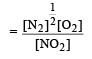
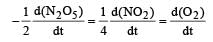
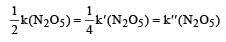
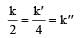
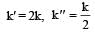
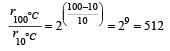
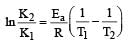
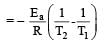
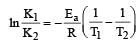
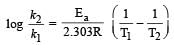
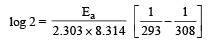
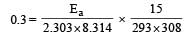
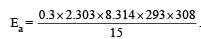
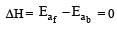
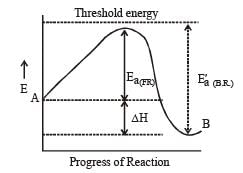
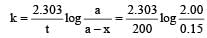
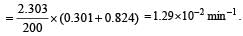
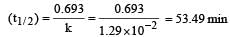
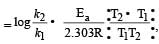 where k1 is the rate constant at temperature T1 and k2 is the rate constant at temperature T2 and Ea is the activation energy. Therefore activation energy of chemical reaction is determined by evaluating rate constant at two different temperatures.
where k1 is the rate constant at temperature T1 and k2 is the rate constant at temperature T2 and Ea is the activation energy. Therefore activation energy of chemical reaction is determined by evaluating rate constant at two different temperatures.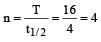
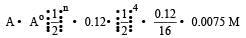
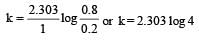
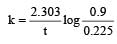
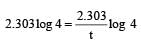
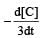 will not represent the reaction rate. It should not have –ve sign as it is product. since
will not represent the reaction rate. It should not have –ve sign as it is product. since 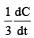 show the rate of formation ofproduct C which will be positive.
show the rate of formation ofproduct C which will be positive.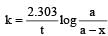
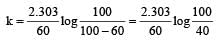
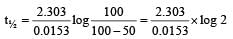
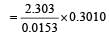 = 45.31 min.
= 45.31 min.