R.C. Mukherjee Test: The Solid State (Old NCERT) - NEET MCQ
30 Questions MCQ Test Chemistry Class 12 - R.C. Mukherjee Test: The Solid State (Old NCERT)
How many number of atoms are there in a cube based unit cell having one atom on each corner and two atoms on each body diagonal of cube -
A metal crystallizes with a face-centered cubic lattice. The edge of the unit cell is 408 pm. The diameter of the metal atom is
The number of atoms/molecules contained in one face centred cubic unit cell of a mono atomic substance is-
In a face centred cubic arrangement of A & B atoms whose A atoms are at the corner of the unit cell & B atoms at the face centres. One of the A atom is missing from one corner in unit cell. The simplest formula of compound is-
A solid has a b.c.c. structure. If the distance of closest approach between the two atoms is 1.73 Å. The edge length of the cell is-
In a close packed array of N spheres, the number of tetrahedral holes are-
In a face centred cubic cell, an atom at the face centre is shared by-
A solid XY has NaCl structure. If radius of X+ is 100 pm. What is the radius of Y- ion-
How many atoms are there in a unit cell of Mg which forms hexagonal crystals, there being a face-centred atom in each end of the unit cell and 3 completely enclosed atoms within the unit cell-
A solid is made of two elements X and Z. The atoms Z are in c.c.p. arrangement while atoms X occupy all the tetrahedral sites. What is the formula of the compound-
Close packing is maximum in the crystal-
The vacant space in b.c.c. unit cell is-
AB crystallizes in a body centred cubic lattice with edge length 'a' equal to 387 pm. The distance between two oppositely charged ions in the lattice is
The unit cell cube length for LiCl (just like NaCl structure) is 5.14 Å. Assuming anion-anion contact, the ionic radius for chloride ion is-
At room temperature, sodium crystallizes in a body centred cubic lattice with a = 4.24 Å. The theoretical density of sodium (At. wt. of Na = 23) is-
The number of octahedral void(s) per atom present in a cubic close-packed structure is
[CBSE AIPMT 2012]
In a face centred cubic lattice the number of nearest neighbour for a given lattice point are-
AB crystallizes in a body centred cubic lattice with edge length 'a' equal to 387 pm. The distance between two oppositively charged ions in the lattice is
A solid AB has rock salt structure. If the edge length is 520 pm and radius of A+ is 80 pm, the radius of anion B- would be -
A binary solid (A+ B-) has a zinc blende structure with B¯ ions constituting the lattice and A+ ions occupying 25% tetrahedral holes. The formula of solid is-
A certain metal crystallises in a simple cubic structure. At a certain temperature, it arranges to give a body centred structure. In this transition, the density of the metal-
For the structure given below the site marked as S is a-
A solid having no definite shape is called-
The mass of a unit cell of CsCl corresponds to-
Close packing is maximum in the crystal lattice of-
The structure of MgO is similar to NaCl. The coordination number of Mg is-
Each unit cell of NaCl consists of 14 chlorine atoms and-
|
108 videos|287 docs|122 tests
|


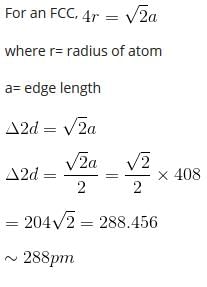

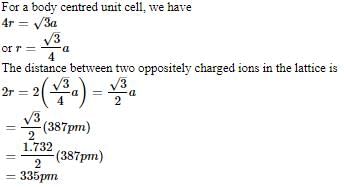 option B
option B 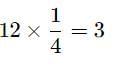
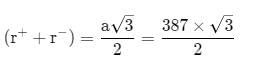 = 335.15 pm
= 335.15 pm











