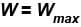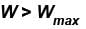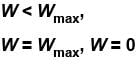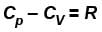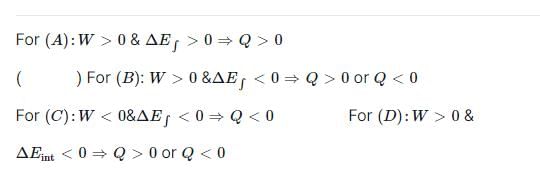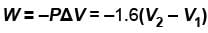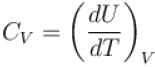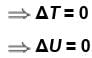First Law Of Thermodynamics MSQ - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - First Law Of Thermodynamics MSQ
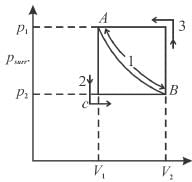
In case of isothermal expansion, which of the following are not forbidden?
Select one or more:
Select one or more:
A thermos bottle containing coffee is vigorously shaken and thereby the temperature of coffee rises. Regard the coffee as the system.
Select one or more:
Select one or more:
Which type of ideal gas will have largest value of CP – CV?
Select one or more:
Select one or more:
Which among the following statements are correct?
Select one or more:
Which of the following processes must violate the first law of thermodynamics?
Select one or more:
1 mole of ideal monoatomic gas at 27ºC expands under adiabatic conditions at a pressure of 1.5 atm from a volume of 4dm3 to 16dm3.
Select one or more:
In an isothermal expansion of an ideal gas,
Select one or more:
Which of the following are path function?
Select one or more:
A resistor immersed in running water carries an electric current. Consider the resistor as the system.
Select one or more:
Consider that 214 J of work done on a system, and 293 J of heat are extracted from the system. Then
Select one or more: