Test: Analysis of Chemical Composition of Biomolecules - ACT MCQ
Test Description
10 Questions MCQ Test - Test: Analysis of Chemical Composition of Biomolecules
Test: Analysis of Chemical Composition of Biomolecules for ACT 2025 is part of ACT preparation. The Test: Analysis of Chemical Composition of Biomolecules questions and answers have been prepared
according to the ACT exam syllabus.The Test: Analysis of Chemical Composition of Biomolecules MCQs are made for ACT 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Analysis of Chemical Composition of Biomolecules below.
Solutions of Test: Analysis of Chemical Composition of Biomolecules questions in English are available as part of our course for ACT & Test: Analysis of Chemical Composition of Biomolecules solutions in
Hindi for ACT course.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free. Attempt Test: Analysis of Chemical Composition of Biomolecules | 10 questions in 15 minutes | Mock test for ACT preparation | Free important questions MCQ to study for ACT Exam | Download free PDF with solutions
Test: Analysis of Chemical Composition of Biomolecules - Question 1
Identify the name of structure
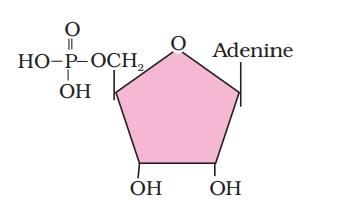

Detailed Solution for Test: Analysis of Chemical Composition of Biomolecules - Question 1
Test: Analysis of Chemical Composition of Biomolecules - Question 2
Which of the following is not a component found in the ash of burnt living tissue?
Detailed Solution for Test: Analysis of Chemical Composition of Biomolecules - Question 2
Test: Analysis of Chemical Composition of Biomolecules - Question 3
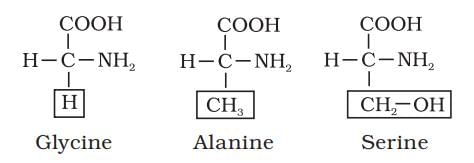
What type of amino acid has its R group as a methyl group?
Detailed Solution for Test: Analysis of Chemical Composition of Biomolecules - Question 3
Test: Analysis of Chemical Composition of Biomolecules - Question 4
Which fraction consists of larger, insoluble compounds after the straining process?
Detailed Solution for Test: Analysis of Chemical Composition of Biomolecules - Question 4
Test: Analysis of Chemical Composition of Biomolecules - Question 5
Which type of lipid is glycerol classified as?
Detailed Solution for Test: Analysis of Chemical Composition of Biomolecules - Question 5
Test: Analysis of Chemical Composition of Biomolecules - Question 6
What are nucleosides that include adenine and a sugar molecule called?
Detailed Solution for Test: Analysis of Chemical Composition of Biomolecules - Question 6
Test: Analysis of Chemical Composition of Biomolecules - Question 7
What is the term used to describe the liquid obtained after straining the ground tissue mixture?
Detailed Solution for Test: Analysis of Chemical Composition of Biomolecules - Question 7
Test: Analysis of Chemical Composition of Biomolecules - Question 8
Identify the name of amino acid through its structure
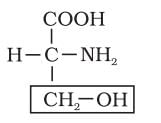
Detailed Solution for Test: Analysis of Chemical Composition of Biomolecules - Question 8
Test: Analysis of Chemical Composition of Biomolecules - Question 9
What remains after completely burning the dry weight of a tissue sample?
Detailed Solution for Test: Analysis of Chemical Composition of Biomolecules - Question 9
Test: Analysis of Chemical Composition of Biomolecules - Question 10
What is used to grind living tissue for chemical analysis in the described procedure?
Detailed Solution for Test: Analysis of Chemical Composition of Biomolecules - Question 10
Information about Test: Analysis of Chemical Composition of Biomolecules Page
In this test you can find the Exam questions for Test: Analysis of Chemical Composition of Biomolecules solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Analysis of Chemical Composition of Biomolecules , EduRev gives you an ample number of Online tests for practice
Download as PDF