IIT JAM Biotechnology Past Year Papers - 2015 - IIT JAM MCQ
30 Questions MCQ Test - IIT JAM Biotechnology Past Year Papers - 2015
Which one of the following most accurately describes the process of natural selection?
Identify the statement that is TRUE of operons.
Signaling pathways usually comprise of several intermediate steps that are arranged in the form of a cascade. What is the primary outcome of such an arrangement?
Which among the following contain(s) oxygen-rich blood in the human vascular system?
I. Right ventricle
II. Aorta
III. Pulmonary vein
Choose the option that lists the correct sequence of steps involved in gene therapy.
P. Injection of expression vector into patient
Q. Wild-type gene is inserted into expression vector
R. Wild-type gene is isolated and cloned
S. Wild-type gene is transcribed and translated in the patient
Cephalin, a biological surfactant, is
The major product(s) produced by gas phase UV irradiation of 2-pentanone is (are)
If a projectile lifts off from the surface of the Earth with a speed of 11.2 km.s-1, then it can escape from the Earth’s gravitational field completely. This is called the escape velocity. If the radius of the Earth were 2 times larger and the mass 8 times larger, then the escape velocity (in km.s-1) would be
The speed of an electron (v), in the lowest energy orbit in the Bohr model of the Hydrogen atom divided by the speed of light in vacuum (c), is given by (where m is the mass of the electron, M is the mass of the proton, ε0 is the permittivity of free space, a0 is the Bohr radius)
Let 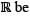 the set of all real numbers. Consider the sets P = {x∈
the set of all real numbers. Consider the sets P = {x∈ 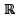 ∶ (x -1)(x2 + 1) = 0}, Q = {x ∈
∶ (x -1)(x2 + 1) = 0}, Q = {x ∈ 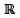 : x2 - 9x + 2 = 0} and S = {x ∈
: x2 - 9x + 2 = 0} and S = {x ∈ 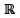 : x = 5y for some y ∈
: x = 5y for some y ∈  }.Then the set (P∩S) ∪ Q contains
}.Then the set (P∩S) ∪ Q contains
In a population growing according to the logistic growth model
Which part of the genomic DNA contains the sequence corresponding to the 5' untranslated region (5' UTR)?
Which one of the following is NOT TRUE of RNA polymerase II?
Choose the option that shows the correct pairing of the cellular components with their corresponding function.

An enzyme shows highest activity in the pH range 2.0 - 3.0. At pH 4.0 and pH 7.0, the enzyme exhibits 50% and 1%, respectively, of its highest activity. Which of the following states of an amino acid residue in the catalytic site is most responsible for its activity profile?
The specific productivity (qp) of cellulase production by Aspergillus niger follows a linear relationship with the specific growth rate (µ) and is of the form qp = αµ + β, where α and β are constants. Assuming that the values of α and β are 0.006 and 25, respectively, which type of product formation kinetics is TRUE?
Choose the option that shows the correct pairing of the diseases with their corresponding causative organisms.
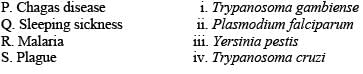
Choose the option that shows the correct pairing of the products with their corresponding microorganisms.

Determine the correctness or otherwise of the following Assertion [a] and Reason [r].
Assertion [a]. B cells secrete antibodies against a virus while cytotoxic T cells kill virus-infected cells.
Reason [r]. B cells confer active immunity while cytotoxic T cells confer passive immunity.
Which one of the following options shows the correct pairing of the enzyme with its corresponding application?

The rate constant for the reaction O (g) + O3 (g) → 2O2 (g) is 8.0 × 10–15 cm3 molecule–1 s–1. The rate constant in dm3 mol–1 s–1, would be
The UV spectrum of 2-butanone and the UV spectrum of methyl vinyl ketone (MVK) are independently recorded and compared. Among the various λmax, 185 nm, 219 nm, 277 nm and 324 nm, the absorption at λmax = 324 nm is due to
The compound meso 2,3-dibromobutane is obtained by
The major product in the following reaction is
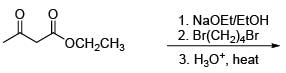
An archaeological sample (remains of an animal) containing 14C isotope of Carbon is found to give 10 beta decays per minute per gram of Carbon. It is known that the natural abundance of 14C in organic matter that is in equilibrium with the atmosphere today will give 15 beta decays per minute per gram of Carbon. The half life of 14C is known to be 5730 years. The estimated age of the sample (in years) is
The minimum light intensity that the human eye can perceive is 10-10 Wm 2. The area of the opening of our eye (the pupil) is approximately 0.4 cm-2 Consider yellow light with wavelength λ = 600 nm. The number of photons incident on the retina per second at the minimum intensity for the eye to respond is
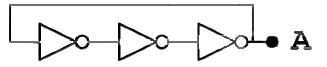
Three NOT gates are connected in senes and the output of the last gate is fed back to the input of the first one as shown in the figure. Each gate has a propagation delay of Td = 1 nano second, which means that the gate requires 1 nano second to change the output after the signal amves at the input. What is the expected output at point A?
Let nCr denote the number 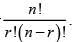 Then for n = 100, the sum of the series
Then for n = 100, the sum of the series
1 - nC1 + nC2 - nC3 + ......... + (- 1)r nCr + ...... ..... + (- 1)n nCn is
The lengths of two sides of a triangle are 2 units and 3 units and the angle included by these two sides is 60o . The length of the third side of the triangle will be
If A and B are two skew-symmetric matrices, the matrix AB + BA must be



















