Test: Basic concept, Zeroth law - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Basic concept, Zeroth law
Among the following the state function(s) is (are)
(i) Internal energy
(ii) Irreversible expansion work
(iii) Reversible expansion work
(iv) Molar enthalpy
(i) Internal energy
(ii) Irreversible expansion work
(iii) Reversible expansion work
(iv) Molar enthalpy
For a spontaneous process, the correct statement(s) is (are)
The number of extensive and intensive properties in the following list is respectively Mass, temperature, pressure, enthalpy, heat capacity, internal energy, density
One mole of an ideal gas at 300 K is expanded isothermally from an initial volume of 1 litre to 10 litres. The ΔE for this process is (R = 2cal mol−1 K−1)
A cyclic process  is shown in
is shown in  diagram for an ideal gas. Which of the following diagram represents the same process?
diagram for an ideal gas. Which of the following diagram represents the same process?
Which of the following conditions are not suitable for a spontaneous reaction?
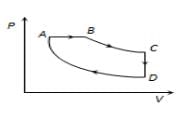
A heat engine carries one mole of an ideal mono-atomic gas around the cycle as shown in the figure. Select the correctoption:
A gas undergoes change from state  to state
to state 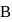 . In this process, the heat absorbed and work done by the gas is
. In this process, the heat absorbed and work done by the gas is 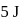 and
and 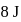 , respectively. Now gas is brought back to
, respectively. Now gas is brought back to 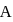 by another process during which
by another process during which 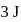 of heat is evolved. In this reverse process of
of heat is evolved. In this reverse process of 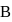 to
to  :
:
A diatomic ideal gas undergoes a thermodynamic change according to the P-V diagram shown in the figure. The total heat given to the gas is nearly
(use ln 2 = 0.7)
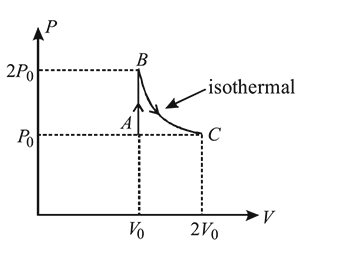
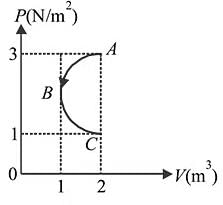
In  diagram shown in figure
diagram shown in figure  is a semicircle. The work done in the process
is a semicircle. The work done in the process  is
is
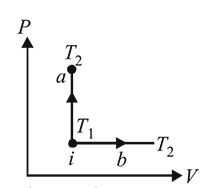
An ideal gas has temperature T1 at the initial state i shown in the P−V diagram. The gas has a higher temperature T2 at the final states a and b, which it can reach the paths shown. The change in entropy:
An ideal monatomic gas is taken round the cycle  as shown in the fig. The work done during the cycle is
as shown in the fig. The work done during the cycle is
Enthalpy change for freezing of 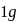 of water at
of water at  and
and 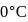 is
is 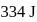 . Calculate the internal energy change in
. Calculate the internal energy change in 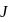 when
when 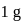 of water is converted into ice?
of water is converted into ice?
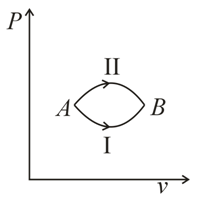
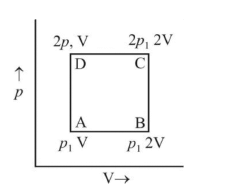
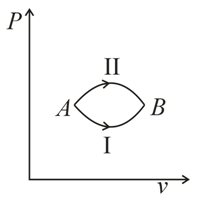
A system goes from 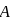 to
to 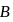 via two processes
via two processes 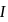 and
and 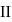 shown in the figure. If
shown in the figure. If 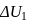 and
and 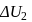 are the changes in internal energies in the processes I and Il respectively
are the changes in internal energies in the processes I and Il respectively
|
353 videos|587 docs|309 tests
|


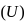 is a state function. It's value depends only on the initial and final positions.
is a state function. It's value depends only on the initial and final positions.
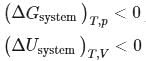

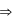 Mass, enthalpy, heat capacity, internal energy.
Mass, enthalpy, heat capacity, internal energy.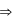 Temperature, pressure, density
Temperature, pressure, density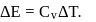 For an isothermal process
For an isothermal process 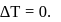 Hence
Hence 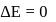


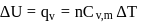
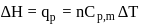
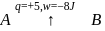
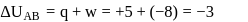

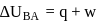
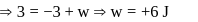 (work done on the system).
(work done on the system).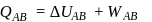
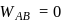
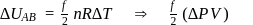
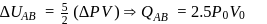
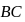 :
: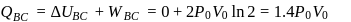
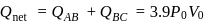
 1)
1) 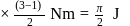
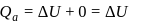 and
and 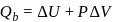
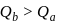
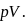
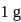 water is converted into ice then internal energy is equal to enthalpy change for freezing at 1 bar and
water is converted into ice then internal energy is equal to enthalpy change for freezing at 1 bar and 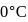 .
.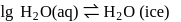
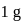 of water at 1 bar and
of water at 1 bar and 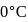 temperature is
temperature is 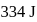 .
.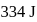 .
.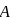 to
to 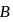 via two processes
via two processes 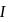 and
and 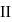 shown in the figure. If
shown in the figure. If 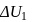 and
and 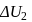 are the changes in internal energies in the processes I and Il respectively
are the changes in internal energies in the processes I and Il respectively