Test: Heat Capacity - NEET MCQ
17 Questions MCQ Test Chemistry Class 11 - Test: Heat Capacity
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Specific heat of iron at 25°C and 1.00 bar is
A rather soft, silvery metal was observed to have a specific heat of 0.225 cal K-1g-1. Thus, metal is
Specific heat of water is 4.184 JK-1g-1, Rise in temperature when 1 kJ of heat is absorbed by 2 moles of H2O is
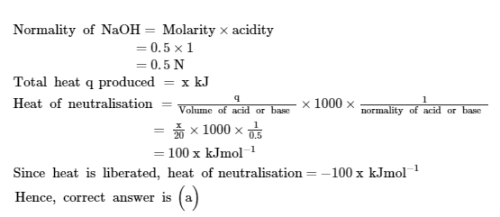
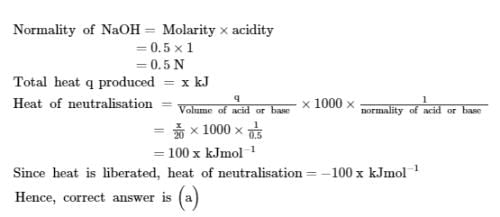
The amount of the heat released when 20 ml 0.5 M NaOH is mixed with 100 ml 0.1 M HCl is x kJ. The heat of neutralization is
The heat capacity ratio, r was determined for cyanogen as 1.177. Thus, Cp for this gas is
Assuming the composition of air to be X (N2) = 0.80, X (O2) = 0.18 and X (CO2) = 0.02, molar heat capacity of air at constant pressure is
Latent heat of fusion of ice is 6.02 kJ mol-1. The heat capacity of water is 4.18 Jg-1K-1. 500 g of liquid water is to be cooled from 20°C to 0°C . Number of ice cubes (each of one mole) required is
Sulphur (2.56 g) is burned in a constant volume calorimeter with excess O2(g). The temperature increases from 21.25°C to 26.72°C . The bomb has a heat capacity of 923 JK-1. Calorimeter contains 815 g of water. Thus, change in internal energy per mole of SO2 formed for the reaction is
(specific heat of water is 4.184 JK-1g-1.)
Direction (Q. Nos. 9) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.
Q.
Statement I : Cv value of helium (He) is always 3/2R but Cv value of hydrogen (H2) is 3/2R at low temperature, 5/2R at moderate temperature and more than 5/2R at higher temperature.
Statement II : At lower temperature, only translational degree of freedom contributes to heat capacity while at higher temperature, rotational and vibrational degrees of freedom also contribute to heat capacity.
Direction (Q. Nos. 10-11) This section contains 2 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Select the correct alternate(s).
Direction (Q. Nos. 12 - 14) This section contains 3 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Q. An 88.5 g piece of iron whose temperature is 352.0 K is placed in a beaker containing 244 g of water at 292.0 K. When thermal equilibrium is reached, what is the change in the temperature of water?
Specific heat of water and iron are (4.184 Jg-1 K-1) and (0.449 Jg-1 K-1), respectively.
The heat capacity of a calorimeter (commonly called the calorimeter constant) was determined by heating the calorimeter and its content using an electrical heater. If ΔT = 1.221 K as 1.25 A of electricity at 3.26 V was passed through the heater immersed in 137.5 g of water in the calorimeter for 175 s, determine the calorimeter constant (in JK-1). (Specific heat of water at constant pressure is 75.291 JK-1 mol-1).
In a constant volume calorimeter, 3.5 g of a gas (molar mass = 28 g mol-1) was burnt in excess oxygen at 298.0 K. The temperature of the calorimeter was found to increase from 298.0 K to 298.45 K due to oxidation of the gas. Given that the heat capacity of the calorimeter is 2.5 kJ K-1, the numerical value of the heat given out in kJ mol-1 is
[IIT JEE 2009]
Direction (Q. Nos. 15-16) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Boiling point of ethanol (C2H5OH) = 78.29°C
Specific heat of ethanol = 2.44 J-1K-1
Enthalpy of vaporisation = 855 Jg-1
Q. Energy required (in kJ) for the following change is
Boiling point of ethanol (C2H5OH) = 78.29°C
Specific heat of ethanol = 2.44 J-1K-1
Enthalpy of vaporisation = 855 Jg-1
Q. Energy required (in kJ) for the following change is
Direction (Q. Nos. 17) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q. According to the classical equipartition of energy theory, the contributions to the molar heat capacity at constant volume are for each degree of translational freedom,
for each degree of rotational freedom and (R) for each degree of translational freedom. Match the species in Column I with the values of molar heat capacity (Cp or CV) in Column II and select answer from codes given below the table.
|
127 videos|245 docs|87 tests
|