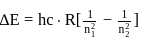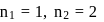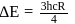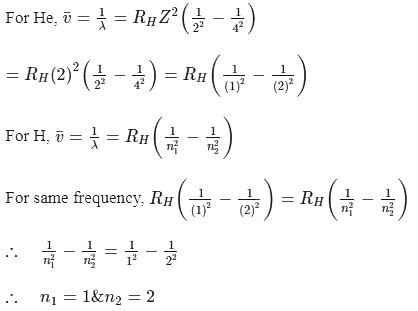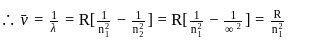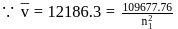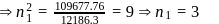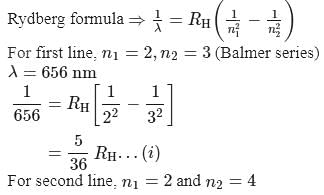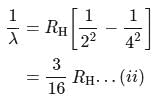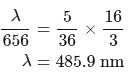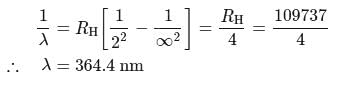Test: Hydrogen spectrum - JEE MCQ
15 Questions MCQ Test - Test: Hydrogen spectrum
The wavelength of Hα line of Balmer series is X Å. What is the wavelength of Hβ line of Balmer series.
A dye absorbs a photon of wavelength 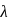 and reemits the same energy into two photons of wavelength
and reemits the same energy into two photons of wavelength 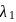 and
and 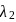 respectively. The wavelength
respectively. The wavelength 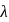 is related with
is related with 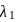 and
and 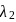 as:
as:
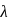 and reemits the same energy into two photons of wavelength
and reemits the same energy into two photons of wavelength 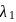 and
and 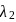 respectively. The wavelength
respectively. The wavelength 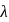 is related with
is related with 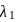 and
and 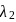 as:
as:The energy of an electromagnetic radiation is 19.875 × 10−13 ergs. What is its wave number in cm−1 ? (h = 6.625 × 10−27 erg sec, c = 3 × 1010 cmsec−1)
What is the maximum wavelength line in the Lyman series of  ion?
ion?
Find the value of wave number 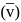 in terms of Rydberg's constant, when transition of electron takes place between two levels of
in terms of Rydberg's constant, when transition of electron takes place between two levels of 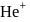 ion whose sum is 4 and difference is 2 .
ion whose sum is 4 and difference is 2 .
The electron energy in hydrogen atom is given by E = (−21.7 × 10−12)/n2 ergs. By calculating the energy required to remove an electron completely from the n = 2 orbit, find out the longest wavelength (in cm) of light that can be used to cause this transition?
The 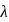 of
of 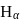 line of Balmer series is
line of Balmer series is 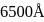 . What is the
. What is the 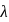 of
of  line of Balmer series?
line of Balmer series?
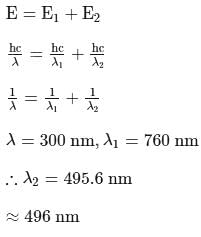
A near UV photon of 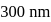 is absorbed by a gas and then re-emitted as two photons. One photon is red with wavelength
is absorbed by a gas and then re-emitted as two photons. One photon is red with wavelength 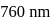 . Hence, wavelength of the second photon is
. Hence, wavelength of the second photon is
What is the lowest energy of the spectral line emitted by the hydrogen atom in the Lyman series?
(h = Planck's constant, c = velocity of light, R = Rydberg's constant).
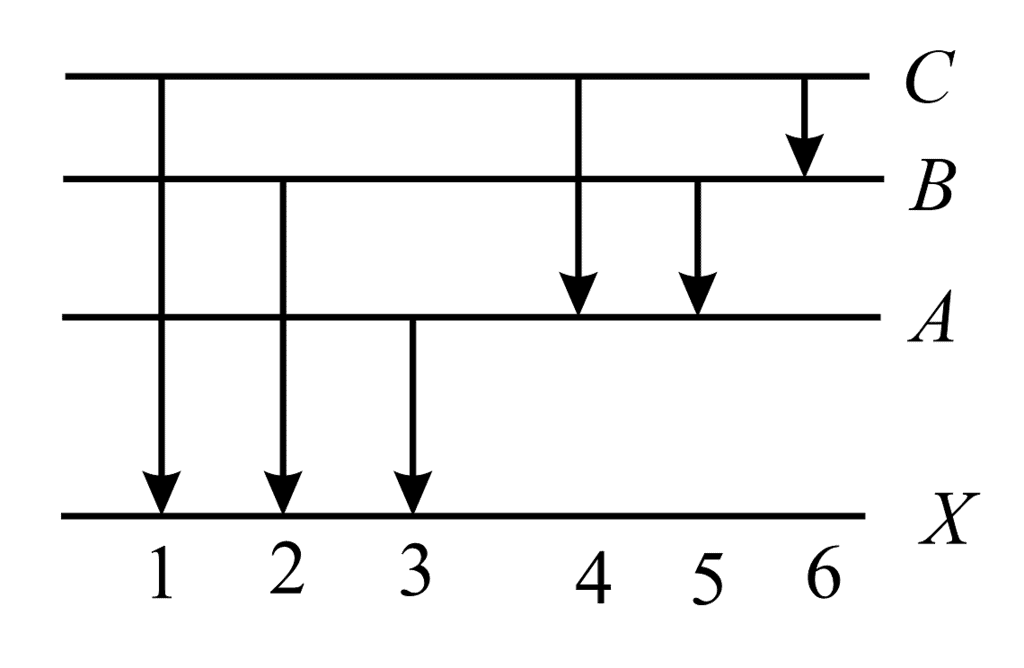
The figure indicates the energy level diagram of an atom and the origin of six spectral lines in emission (e.g. line no. 5 arises from the transition from level B to A). The following spectral lines will also occur in the absorption spectrum
The frequency of light emitted for the transition n = 4 to n = 2 of the He+ is equal to the transition in H atom corresponding to which of the following ?
In hydrogen atomic spectrum, a series limit is found at 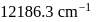 . Then it belong to
. Then it belong to
If the wavelength of the first line in Balmer series is 656 nm, then the wavelength of its second line and limiting line respectively are


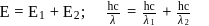
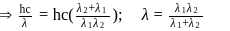

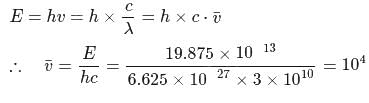
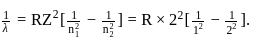
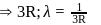
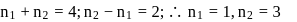
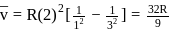
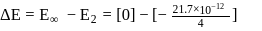
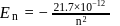 ergs
ergs 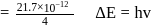
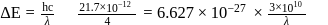
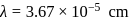
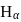 lines of Balmer series
lines of Balmer series 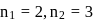
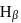 line of Balmer series
line of Balmer series 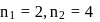
 ...(1)
...(1)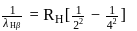 ...(2)
...(2)