Test: Importance & Scope Of Chemistry - NEET MCQ
20 Questions MCQ Test Chemistry Class 11 - Test: Importance & Scope Of Chemistry
The two effective drugs which act as life-saving drugs for cancer therapy and AIDS victims respectively are:
Which of the following statements is/are correct?
The number of significant figures in 3256 is:
A number of significant figures in 0.001520 is:
If the true value for a result is 2.00 g and a student takes 2 measurements and reports the results as 1.95 g and 1.93 g, then we can conclude that:
Out of the numbers: 6.26, 5.8, 0.00267, 0.03, the one with the least significant figures is:
If the true value for a result is 3.00 m and a student records two readings as 3.01 m and 2.99 m, then we can conclude that:
The number of significant figures in 3.1205 are:
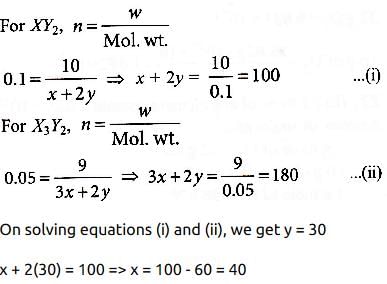
Suppose the elements X and Y combine to form two compounds XY2 and X3Y2. When 0.1 mole of XY2 weighs 10 g and 0.05 mole of X3Y2 weighs 9 g, the atomic weights of X and Y are:
If we add the numbers 6.68 and 0.8, the result will be:
Molecular mass of glucose molecule (C6H12O6) is:
|
127 videos|245 docs|87 tests
|