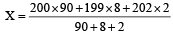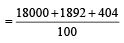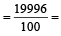31 Year NEET Previous Year Questions: Some Basic Concepts Of Chemistry - 1 - NEET MCQ
20 Questions MCQ Test - 31 Year NEET Previous Year Questions: Some Basic Concepts Of Chemistry - 1
An organic compound contains 80% (by wt.) carbon and the remaining percentage of hydrogen. The right option for the empirical formula of this compound is: [Atomic wt. of C is 12, H is 1] (2021)
Which one of the followings has maximum number of atoms? (2020)
In an experiment it showed that 10 mL of 0.05 M solution of chloride required 10 mL of 0.1 M solution of AgNO3, which of the following will be the formula of the chloride (X stands for the symbol of the element other than chlorine): [NEET Kar. 2013]
6.02 × 1020 molecules of urea are present in 100 mL of its solution. The concentration of solution is: [NEET 2013]
Which has the maximum number of molecules among the following ? [2011 M]
The number of atoms in 0.1 mole of a triatomic gas is: [2010] (NA = 6.02 ×1023 mol–1)
What is the [OH–] in the final solution prepared by mixing 20.0 mL of 0.050 M HCl with 30.0 mL of 0.10 M Ba(OH)2? [2009]
How many moles of lead (II) chloride will be formed from a reaction between 6.5 g of PbO and 3.2 g of HCl ? [2008]
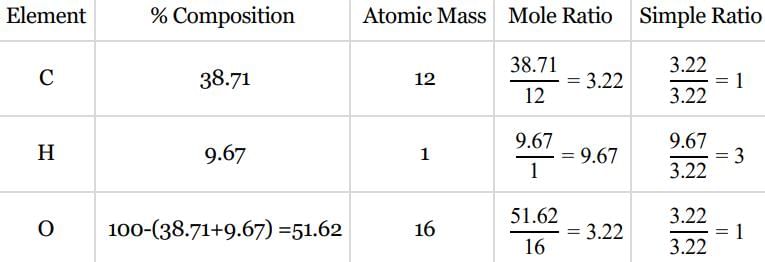
An organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gave C, 38.71% and H, 9.67%. The empirical formula of the compound would be: [2008]
What volume of oxygen gas (O2) measured at 0°C and 1 atm, is needed to burn completely 1L of propane gas (C3H8) measured under the same conditions? [2008]
Number of moles of MnO4- required to oxidize one mole of ferrous oxalate completely in acidic medium will be : [2008]
Volume occupied by one molecule of water (density = 1 g cm–3) is : [2008]
The number of moles of KMnO4 that will be needed to react with one mole of sulphite ion in acidic solution is [2007]
Concentrated aqueous sulphuric acid is 98% H2SO4 by mass and has a density of 1.80 g mL– 1. Volume of acid required to make one litre of 0.1MH2SO4 solution is [2007]
The number of moles of KMnO4 reduced by one mole of KI in alkaline medium is: [2005]
The mass of carbon anode consumed (givin g only carbondioxide) in the production of 270 kg of aluminium metal from bauxite by the Hall process is (Atomic mass: Al = 27) [2005]
In Haber process 30 litres of dihydrogen and 30 litres of dinitrogen were taken for reaction which yielded only 50% of the expected product. What will be the composition of gaseous mixture under the aforesaid condition in the end? [2003]
The maximum number of molecules is present in:
An element , X has the following isotopic composition :
200X:90 %
199X : 8.0%
202X ; 2.0 %
The weighted average atomic mass of the naturally occuring element X is closest to
10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be:



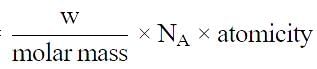
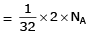
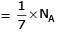
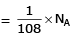
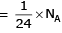

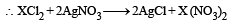
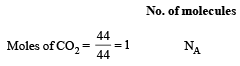
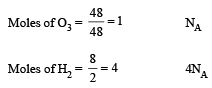
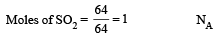
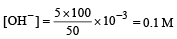

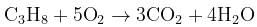
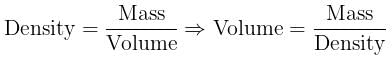
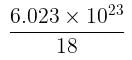 molecules of water = 1 cm3 [∵ 1g water = 1/18moles of water]
molecules of water = 1 cm3 [∵ 1g water = 1/18moles of water]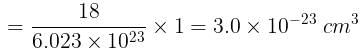
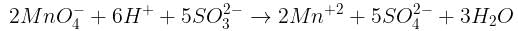
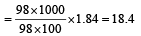
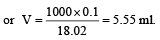
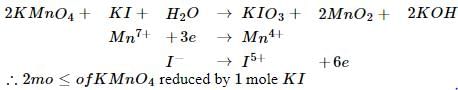
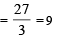
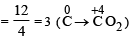
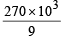 = 30 × 103
= 30 × 103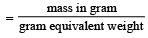
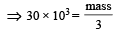
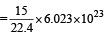 molecules
molecules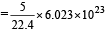 molecules
molecules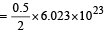 molecules
molecules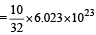 molecules
molecules