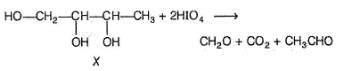Test: Tests & Distinction of Alcohols - NEET MCQ
20 Questions MCQ Test Topic-wise MCQ Tests for NEET - Test: Tests & Distinction of Alcohols
Which of the following tests can be used to distinguish between two isomeric ketones: 3- pentanone and 2- pentanone?
An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X is
An organic compound C4H10O (X) on reaction with I2/red-P give s C4H9I which on further reaction with AgNO2 gives C4H9NO2 (Y). Y on treatment with HNO2 forms a blue solution which turns to red on making solution slightly alkaline. The possible identity of X is
An organic compound X (C7H16O) on treatm ent with concentrated HCI solution forms immediate turbidity even in the absence of ZnCl2. Also, X is resolvable into enantiomers. The correct statement concerning X is
Which reagent given below does not produce any visible change when added to ethylene glycol?
Which of the following is least likely to form turbidity with HCI in the presence of ZnCI2 at room temperature?
The correct statement regarding 3-ethyl-3-hexanol is
An alcohol has molecular formula C6H12O X and it gives immediate turbidity with cold, concentrated HCI even in the absence of ZnCI2. X can also be obtained by treatment of an ether with excess of CH3MgBr followed by acid hydrolysis. Hence, the correct statement regarding X is
One or More than One Options Correct Type
Direction (Q. Nos. 9-12) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Which reagent(s) can be used to differentiate between 2-pentanol and 1-pentanol?
Alcohols given below that behaves like 1°-aliphatic alcohol in Lucas test is/are
Consider the following reaction sequence,
R — OH 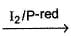 R — I
R — I 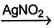 R—NO2
R—NO2 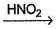 Blue
Blue
Hence, R—OH could be
Consider the following reaction,
The correct statement(s) concerning X and Y is/are
Comprehension Type
Direction (Q. Nos. 13-15) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Passage
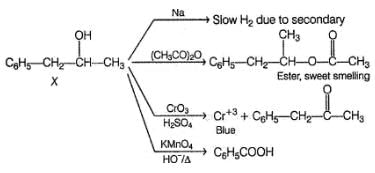
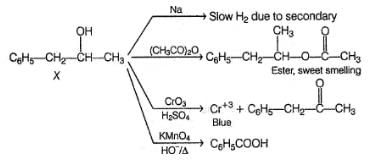
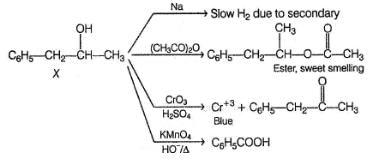
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
The structure of X is
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
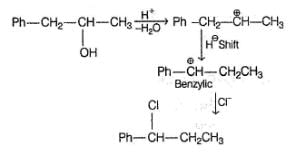
If X is treated with HCI in the presence of ZnCI2, the major product would be
An organic compound X (C9H12O) gives the following reactions :
i. Na - Slow gas bubble formation
ii. Acetic anhydride - Pleasent smelling liquid
iii. CrO3-H2SO4 - Blue-green solution
iv. Hot KMnO4 - Benzoic acid
v. Br2-CCI4 - No decolouration
vi. I2 + NaOH - Yellow solid is formed
vii. X rotates the plane polarised light
Q.
An isomer of X, Y was also found to be optically active. It showed the same reactions as X except (vi). Hence, Y is
One Integer Value Correct Type
Direction (Q. Nos. 16-19) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
An organic compound X (C5H12O) is chiral. Also
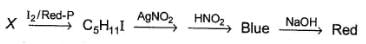
Q.
How many primary hydrogens are present in X?
An alcohol X (C7H16O) gives immediate turbidity with HCI/ZnCI2 and can be resolved into enantiomers. Also X has a tertiary hydrogen. How many CH3— groups are present in X?
An alcohol X (C4H10O3) is chiral and absorbs two moles of HIO4 per mole of X. How many stereoisomers exist for X?
An organic compound X (C7H16O) gives effervescence with Na. X has both enantiomers and diastereomers. X gives yellow precipitate with alkaline iodine solution. With CrO3 - H2SO4 , X is converted into C7H14O (Y) which has enantiom ers but not diastereom ers. X on refluxing with H2SO4 isomerises to Z which neither gives yellow precipitate with alkaline iodine nor changes colour of CrO3 - H2SO4 . What is the minim um number of carbons that can be present in the parent chain of X ?
Which of the following reagents can be used to distinguish phenol from a primary alcohol?
|
9 docs|1256 tests
|