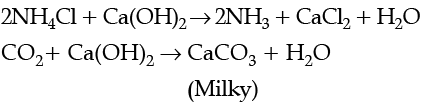Assertion & Reason Test: The s-Block Elements (Old NCERT) - NEET MCQ
10 Questions MCQ Test NCERT Based Tests for NEET - Assertion & Reason Test: The s-Block Elements (Old NCERT)
In the following questions more than one option of column I and II may be correlated. Match the elements given in Column I with the properties mentioned in Column II.
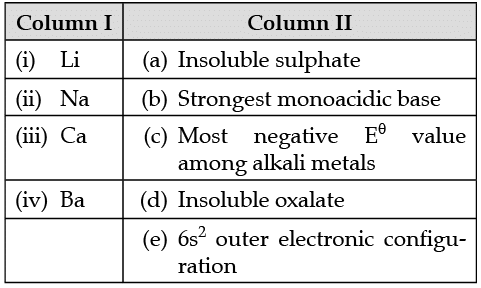

In the following questions more than one option of column I and II may be correlated. Match the elements given in Column I with the properties mentioned in Column II.
Match the compounds given in Column I with their uses mentioned in Column II.
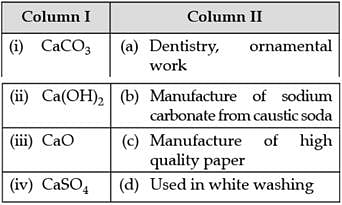

In the following questions more than one option of column I and II may be correlated.
Match the elements given in Column I with the properties mentioned in Column II. Match the compounds given in Column I with their uses mentioned in Column II.

Match the elements given in Column I with the properties mentioned in Column II. Match the compounds given in Column I with their uses mentioned in Column II.

In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): The carbonate of lithium decomposes easily on heating to form lithium oxide and CO2.
Reason (R): Lithium being very small in size polarises large carbonate ion leading to the formation of more stable Li2O and CO2.
In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A) : Beryllium carbonate is kept in the atmosphere of carbon dioxide.
Reason (R) : Beryllium carbonate is unstable and decomposes to give beryllium oxide and carbon dioxide.
Choose the correct statements from the following.
(i) Beryllium is not readily attacked by acids because of the presence of an oxide film on the surface of the metal.
(ii) Beryllium sulphate is readily soluble in water as the greater hydration enthalpy of Be2+ overcomes the lattice enthalpy factor.
(iii) Beryllium exhibits coordination number more than four.
(iii) Beryllium oxide is purely acidic in nature.
Which of the following are the correct reasons for anomalous behaviour of lithium?
(i) Exceptionally small size of its atom
(ii) Its high polarising power
(iii) It has high degree of hydration
(iv) Exceptionally low ionisation enthalpy
When Zeolite, which is hydrated sodium aluminium silicate is treated with hard water, the sodium ions are exchanged with which of the following ion(s)?
(i) H+ ions
(ii) Mg2+ ions
(iii) Ca2+ ions
(iv) SO42– ions
Amphoteric hydroxides react with both alkalies and acids. Which of the following Group 2 metal hydroxides is soluble in sodium hydroxide?
A chemical A is used for the preparation of washing soda to recover ammonia. When CO2 is bubbled through an aqueous solution of A, the solution turns milky. It is used in white washing due to disinfectant nature. What is the chemical formula of A?
|
748 tests
|