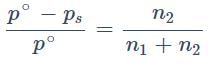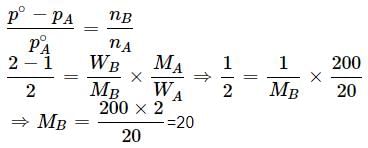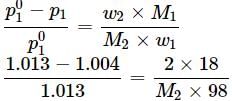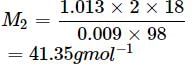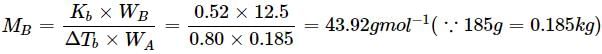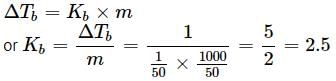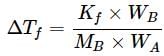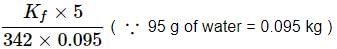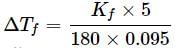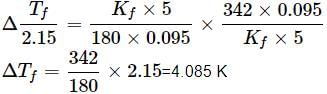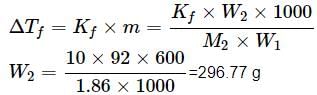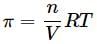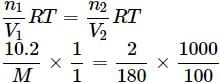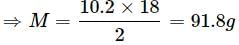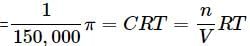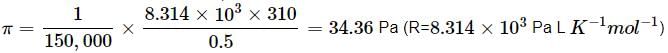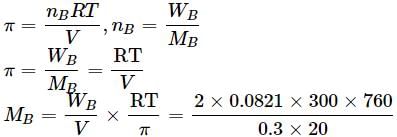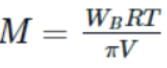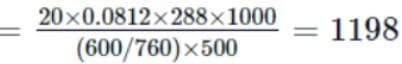Test: Colligative Properties and Determination of Molar Mass (NCERT) - NEET MCQ
25 Questions MCQ Test - Test: Colligative Properties and Determination of Molar Mass (NCERT)
The relative lowering in vapour pressure is proportional to the ratio of number of
Vapour pressure of a pure liquid X is 2 atm at 300 K. It is lowered to 1 atm on dissolving 1 g of Y in 20 g of liquid X. If molar mass of X is 200, what is the molar mass of Y?
An aqueous solution of 2% non - volatile solute exerts a pressure of 1.004 bar at the normal boiling point of the solvent. What is the molar mass of the solute?
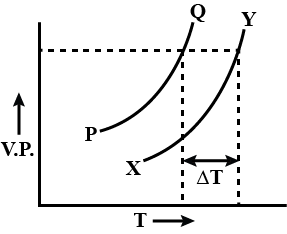
In the graph plotted between vapour pressure (V.P) and temperature (T).
A solution containing 12.5g of non-electrolyte substance in 185g of water shows boiling point elevation of 0.80K. Calculate the molar mass of the substance. (Kb = 0.52K kg mol−1)
If 1g of solute (molar mass = 50g mol−1) is dissolved in 50g of solvent and the elevation in boiling point is 1K. The molar boiling constant of the solvent is?
2 g of sugar is added to one litre of water to give sugar solution. What is the effect of addition of sugar on the boiling point and freezing point of water?
Sprinkling of salt helps in clearing the snow-covered roads in hills. The phenomenon involved in the process is
Equimolar solutions in the same solvent have
A 5% solution (w/W) of cane sugar (molar mass = 342 g mol-1) has freezing point of 271 K. What will be the freezing point of a 5% glucose (molar mass = 18 g mol-1) in water if freezing point of pure water is 273.15 K?
What weight of glycerol should be added to 600g of water in order to lower its freezing point by 10∘C? (Kf = 1.86∘Cm−1)
The osmotic pressure of a solution can be increased by
Answer the following questions on the basis of given paragraph.
Osmotic pressure is widely used to determine molar masses of proteins and polymers. Two solutions having same osmotic pressure are called isotonic solutions. Water can flow in or out from substance depending on if it is kept in hypotonic or hypertonic solutions. The direction of the osmosis can be reversed if a pressure larger than osmotic pressure is applied on solution side.
Q. People taking lot of salt experience puffiness or swelling of the body due to
Answer the following questions on the basis of given paragraph.
Osmotic pressure is widely used to determine molar masses of proteins and polymers. Two solutions having same osmotic pressure are called isotonic solutions. Water can flow in or out from substance depending on if it is kept in hypotonic or hypertonic solutions. The direction of the osmosis can be reversed if a pressure larger than osmotic pressure is applied on solution side.
Q. The preservation of meat by salting and of fruits by adding sugar protects them from bacterial action because
Answer the following questions on the basis of given paragraph.
Osmotic pressure is widely used to determine molar masses of proteins and polymers. Two solutions having same osmotic pressure are called isotonic solutions. Water can flow in or out from substance depending on if it is kept in hypotonic or hypertonic solutions. The direction of the osmosis can be reversed if a pressure larger than osmotic pressure is applied on solution side.
Q. Sea water is desalinated to get fresh water by which of the following methods?
Which of the following statements is not correct?
10% solution of urea is isotonic with 6% solution of a non-volatile solute X.What is the molecular mass of solute X?
A solution containing 10.2g glycerine per litre is isotonic with a 2% solution of glucose. What is the molecular mass of glycerine?
What will be the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 150,000 in 500 mL of water at 37∘C?
Osmotic pressure of a solution containing 2g dissolved protein per 300cm2 of solution is 20mm of Hg at 27oC. The molecular mass of protein is
A solution is made by dissolving 20g of a substance in 500mL of water. Its osmotic pressure was found to be 600mm of Hg at 15∘C. Find the molecular weight of the substance.
Which of the following statements is not correct?
Grapes placed in three beakers X, Y and Z containing different type of solutions are shown in figures.
If beaker X contains water, Y and Z contains:
A plant cell shrinks when it is kept in a
Which of the following statements is correct?