Test: Diols & Polyols - NEET MCQ
20 Questions MCQ Test - Test: Diols & Polyols
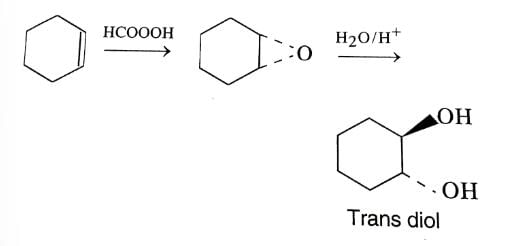
Which is the most suitable condition for the following transformation?
What is the increasing order of solubility of the following in water?
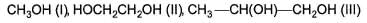

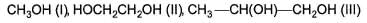

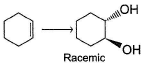
How many different diols product are formed in the following reaction?
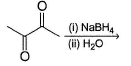
Which does not form dihalide when treated with ethylene glycol?
What is the major organic product of the following reaction?
What is formed as the major product in the reaction given below?
One or More than One Options Correct Type
Direction (Q. Nos. 8-13) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Which of the following reaction(s) gives diols?
In the following rearrangement, reaction possible product(s) is/are
Comprehension Type
Direction (Q. Nos. 14-16) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
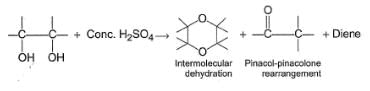
Glycol when heated with concentrated H2SO4, undergo a variety of reactions as

Nature of predominant product depends upon the nature of other groups present at the two α-carbons.
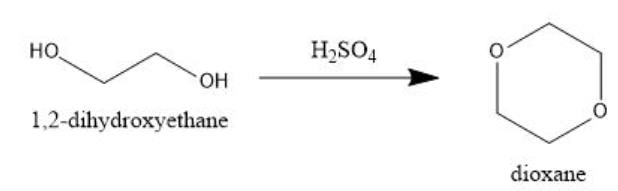
Q. Which is most likely to undergo intermolecular dehydration to give dioxane or substituted dioxane?
Glycol when heated with concentrated H2SO4, undergo a variety of reactions as
Nature of predominant product depends upon the nature of other groups present at the two α-carbons.
Q.
Which of the following is most likely to undergo Pinacol-pinacolone rearrangement?
Glycol when heated with concentrated H2SO4, undergo a variety of reactions as
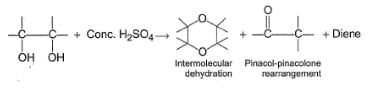
Nature of predominant product depends upon the nature of other groups present at the two α-carbons.
Q.
Which diol given below is most likely to form diene in the above reaction?
Matching List Type
Direction (Q . Nos. 17 and 18) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
Match the reactant from Column I with the reaction(s) from Column II and mark the correct option from the codes given below.
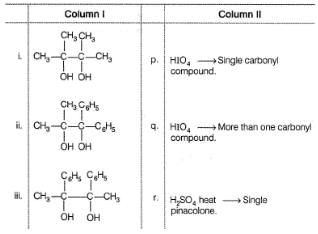
Match the compounds in Column I with their reaction and product(s) in Column II and mark the correct option from the codes given below.

Which of the following methods is the best way to distinguish between a primary alcohol and a diol?
Which of the following reagents converts alkenes directly to vicinal diols?