Test: Enthalpy of Reaction & Formation - NEET MCQ
18 Questions MCQ Test NCERT Based Tests for NEET - Test: Enthalpy of Reaction & Formation
Direction (Q. Nos. 1-9) This section contains 9 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The difference between ΔrH° and ΔrE° (in kcal) for the reaction
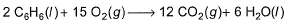
at 298 K in kcal is
The ΔrH° for CO2(g), CO(g) , and H2O(g) are - 393.5°, - 110.5 and - 241.8 kJ mol-1, respectively. Thus, ΔrH° for the reaction (in kJ)
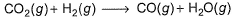
[IITJEE 2000]
Enthalpies of formation of CO(g) , CO2 (g) , N2O (g) and N2O4 (g) are -110, - 393, 81 and 9.7 kJ mol-1. Thus, ΔrU for the reaction at 298 K is,
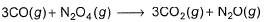
Given
Q. Thus, heat of formation of CH3OH(/)is
Hot carbon reacts with steam to produce an equimolar mixture of CO(g)and H2(g) known as water gas
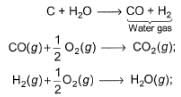
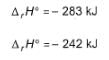
Q.
Energy released , if water gas is used as fuel is
For a gaseous phase reaction
When equilibrium is set up, K = 0.33
Energy involved (in kJ) is
One mole of a non-ideal gas undergoes a change of state (2.0 atm, 3.0 L, 95 K) → (4.0 atm, 5.0 L, 245 K) with a change in internal energy, ΔrE° = 30.0 L atm. The change in enthalpy (ΔrH°) of the process in L-atm is
[IIT JEE 2002]
Which of the following reactions defines ΔfH° ?
Ethanol can undergo decomposition to form two sets of products
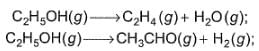
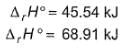
Q.
if the molar ratio of C2H4 to CH3CHO is 8 : 1 in a set of product gases, then, energy involved in the decomposition process is
Direction (Q. No. 10) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.
Q.
Statement I : Based on the following thermodynamic data
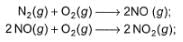
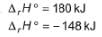
NO2 is more stable than NO.
Statement II : NO (g) is an endothermic compound while, NO2(g) is an exothermic compound.
Direction (Q. Nos. 11-12) This section contains 2 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Given,
Q. Thus, standard heat of formation of
Direction (Q. Nos. 13-15) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d).
Based on the following thermodynamic data,
Q. Which oxidising agent will generate the greatest amount of energy per mole of H2(g)?
Based on the following thermodynamic data,
Q. Which oxidising agent will generate the greatest amount of energy per gram of oxidising agent?
Based on the following thermodynamic data,
Q. On the total mass basis of reactants, which reaction will generate the greatest amount of heat?
Direction (Q. Nos. 16 - 18) This section contains 3 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Q. How much heat (in kcal) is required to convert 36 g of diamond into graphite?
Based on the following reactions,
Q. Heat of formation of NO2 (in kcal) is ........
How many of the following has zero standard molar enthalpy of the formation at 298 K?
[IIT JEE 2010]
|
678 tests
|




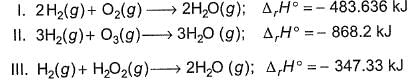
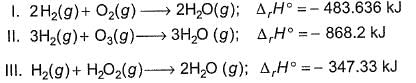 From I, total mass of reactant, 36 gm
From I, total mass of reactant, 36 gm










