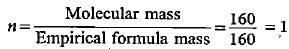NEET Exam > NEET Tests > Chemistry Class 11 > Test: Percentage Composition (NCERT) - NEET MCQ
Test: Percentage Composition (NCERT) - NEET MCQ
Test Description
5 Questions MCQ Test Chemistry Class 11 - Test: Percentage Composition (NCERT)
Test: Percentage Composition (NCERT) for NEET 2025 is part of Chemistry Class 11 preparation. The Test: Percentage Composition (NCERT) questions and answers have been
prepared according to the NEET exam syllabus.The Test: Percentage Composition (NCERT) MCQs are made for NEET 2025 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Percentage Composition (NCERT) below.
Solutions of Test: Percentage Composition (NCERT) questions in English are available as part of our Chemistry Class 11 for NEET & Test: Percentage Composition (NCERT) solutions in
Hindi for Chemistry Class 11 course. Download more important topics, notes, lectures and mock
test series for NEET Exam by signing up for free. Attempt Test: Percentage Composition (NCERT) | 5 questions in 5 minutes | Mock test for NEET preparation | Free important questions MCQ to study Chemistry Class 11 for NEET Exam | Download free PDF with solutions
Test: Percentage Composition (NCERT) - Question 1
0.48 g of a sample of a compound containing boron and oxygen contains 0.192 g of boron and 0.288 gof oxygen. What will be the percentage composition of the compound?
Detailed Solution for Test: Percentage Composition (NCERT) - Question 1
Test: Percentage Composition (NCERT) - Question 2
A compound contains two elements 'x' and 'Y' in the ratio of 50% each. Atomic mass of 'x' is 20 and 'Y' is 40. What can be its simplest formula?
Detailed Solution for Test: Percentage Composition (NCERT) - Question 2
Test: Percentage Composition (NCERT) - Question 3
The empirical formula of a compound is CH2O2.What could be its molecular formula?
Detailed Solution for Test: Percentage Composition (NCERT) - Question 3
Test: Percentage Composition (NCERT) - Question 4
A gas has molecular formula (CH)n. If vapour density of the gas is 39, what should be the formula of the compound?
Detailed Solution for Test: Percentage Composition (NCERT) - Question 4
Test: Percentage Composition (NCERT) - Question 5
Choose the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively and its molecular mass is 160.
Detailed Solution for Test: Percentage Composition (NCERT) - Question 5
|
127 videos|245 docs|87 tests
|
Information about Test: Percentage Composition (NCERT) Page
In this test you can find the Exam questions for Test: Percentage Composition (NCERT) solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Percentage Composition (NCERT), EduRev gives you an ample number of Online tests for practice