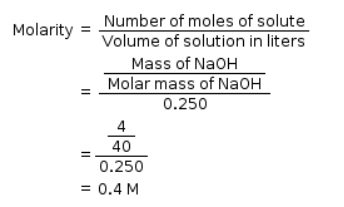Test: Some Basic Concepts Of Chemistry - NEET MCQ
25 Questions MCQ Test Chemistry Class 11 - Test: Some Basic Concepts Of Chemistry
Physical properties are those properties which ______ measured or observed ______ changing the identity or the composition of the substance
SI units for Base Physical Quantities of length, mass and current are
For the reaction
Fe2 O3 (s) + 3 CO (g) → 2 Fe (g) + 3 CO2,
224 g of CO is available to react with 400 g Fe2O3, the yield of iron and CO2, are:
According to the law of conservation of mass, a balanced chemical equation has
A measured temperature is 1000F on Fahrenheit scale, then what is this reading be on Celsius scale:
The calculation of masses (sometimes volumes also) of the reactants and the products involved in a chemical reaction is called
Molarity of NaOH in a solution prepared by dissolving 4 g of NaOH in enough water to form 250 ml of solution is:
The kelvin scale is related to celsius scale by
How many atoms of hydrogen are in 67.2 L of H2 at STP?
In scientific notation for such numbers, any number can be represented in the form N × 10n where
Directions : In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion: In laboratory, a solution of a desired concentration is prepared by diluting a stock solution.
Reason: Stock solution is the solution of higher concentration.
How many atoms of Oxygen are there in 18g of water?
(Hint: Avogadro’s Number = 6.02 x 1023 atoms/mol)
If a matter has definite volume and definite shape, then it is:
The number of moles of solute present in 1 kg of solvent is called:
|
127 videos|245 docs|87 tests
|