Test: Distribution Of Electrons In Atoms - Class 9 MCQ
15 Questions MCQ Test Online MCQ Tests for Class 9 - Test: Distribution Of Electrons In Atoms
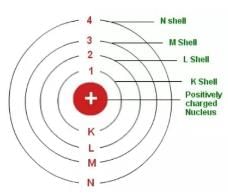
N shell can have a maximum of _______ electrons.
If both K and L shells are filled, the total number of electrons contained in them will be:
Which of the following is the correct electronic configuration for magnesium?
The number of electrons in an atom of Fluorine is 9. Its electronic configuration is _______.
Bohr proposed that while revolving in discrete orbits, the electrons:
The electronic configuration of an atom with atomic number 19 is:
Which of the following elements has the electron configuration 2?
What will be the valency of an element having atomic number (Z) = 7?
Each of the stationary orbits are associated with:
The increasing order of the energy levels in an atom is _______ .
Which one of the following elements has two valence electrons but is a noble gas?
The maximum number of electrons that can be accomodated in an orbit is given by the formula _______ where n is the number of orbit.
If Z = 6, what would be the valency of the element?
How many valence electrons are present in a potassium atom?
|
5 docs|319 tests
|