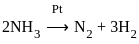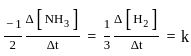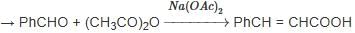Chemistry: CUET Mock Test - 3 - CUET MCQ
30 Questions MCQ Test - Chemistry: CUET Mock Test - 3
The decomposition of NH3 on platinum surface is zero order reaction. If k = 2.5 × 10-4 mol L-1s-1 the rate of production of H2 is
What is the molecularity of the following elementary reaction: 2NO+O2→2NO2?
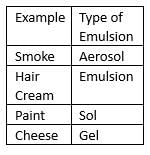
Which one of the following is NOT an example of colloids?
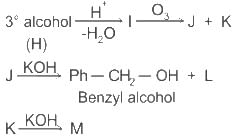
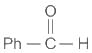
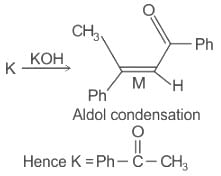
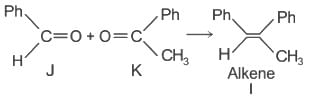
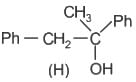
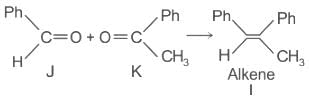
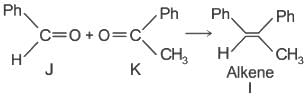
Which of the following statements is correct for compound (K)?
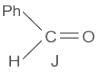
When (J) is treated with acetic anhydride, in the presence of the corresponding salt of an acid, the product obtained is
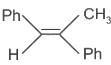
The structure of compounds J, K, and L, respectively, are
Consider the following statements regarding colligative properties:
(A) Colligative properties depend on the nature of the solute.
(B) Colligative properties depend on the number of solute particles.
(C) Osmotic pressure is one of the colligative properties.
(D) Elevation in boiling point and depression in freezing point are examples of colligative properties.
Choose the correct statements:
Consider the following statements regarding Raoult’s law:
(A) Raoult’s law holds true only for ideal solutions.
(B) Raoult’s law states that the vapor pressure of a solvent in a solution is proportional to the mole fraction of the solvent.
(C) Raoult’s law is not valid for solutions with highly volatile solutes.
(D) Raoult's law applies to both ideal and non-ideal solutions.
Choose the correct statements:
Consider the following statements regarding solutions:
(A) Solutions with the same boiling point and freezing point are said to be ideal solutions.
(B) Colligative properties depend on the number of solute particles.
(C) In ideal solutions, intermolecular forces between solvent and solute are the same.
(D) Osmosis is the movement of solvent from lower concentration to higher concentration of solute.
Choose the correct statements:
Consider the following statements regarding the molal freezing point depression constant:
(A) It depends on the solvent used.
(B) It is the same for all solvents.
(C) The value of the molal freezing point depression constant is used to calculate the depression in freezing point.
(D) Molal freezing point depression constant is dependent of the amount of solute.
Choose the correct statements:
The spin only magnetic moment value (in Bohr magneton units) of Cr(CO)6 is
The anion acetylacetonate (acac) forms chelate with Co3+, The ring of the chelate is
Oxidation states of iron in the complexes [Fe(H2O)5NO]2+ are
In electrolysis of a fused salt, the weight deposited on an electrode will not depend on-
The electrolysis of a solution resulted in the formation of H2 at the cathode and Cl2 at the anode. The liquid is-
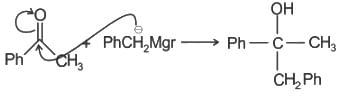
Consider the following reaction and the product formed.
Q.
The most likely mechanism of the above reaction is
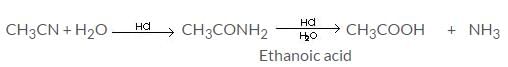
Hydrolysis of methyl cyanides with dil. mineral acids gives:
Which of the following process do not yield alcohols?
What is the coordination number for a two-dimensional square close packed structure?
Voids in two-dimensional hexagonal close packed structure are ___________ in shape.
Which of the following is the correct Gibbs equation?
Which of the following elements does not belong to group 16 of the periodic table?