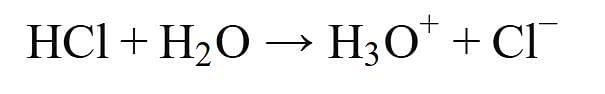Assertion & Reason Test: Acids, Bases & Salts - 1 - Class 10 MCQ
16 Questions MCQ Test Science Class 10 - Assertion & Reason Test: Acids, Bases & Salts - 1
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion: After white washing the walls, a shiny white finish on walls is obtained after two to three days.
Reason: Calcium Oxide reacts with Carbon dioxide to form Calcium Hydrogen Carbonate which gives shiny white finish.
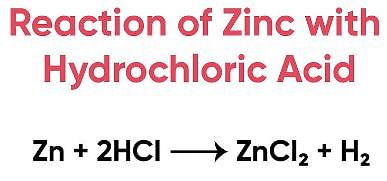
Assertion (A): When zinc is added to dilute hydrochloric acid, hydrogen is given off.
Reason (R): Hydrogen chloride molecules contain hydrochloric acid and hydrogen atoms
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Assertion (A): Gas bubbles are observed when sodium carbonate is added to dilute hydrochloric acid.
Reason (R): Carbon dioxide is given off in the reaction.
Assertion (A): Ammonia solution is an alkali.
Reason (R): Ammonia solution turns blue litmus paper red.
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): When common salt is kept open, it absorbs moisture from the air.
Reason (R): Common salt contains little magnesium chloride.
Assertion (A): Baking soda creates acidity in the stomach.
Reason (R): Baking soda is alkaline.
Assertion (A): Plaster of Paris is used by doctors for setting fractured bones.
Reason (R): When Plaster of Paris is mixed with water and applied around the fractured limbs, it sets into a hard mass.
Direction: In the Following Questions, A Statement of Assertion (A) Is Followed by A Statement of Reason (R). Mark The Correct Choice As:
Assertion: While dissolving an acid or base in water, the acids must always be added slowly to water with constant stirring.
Reason: Dissolving an acid on a base in water is a highly exothermic reaction.
Assertion : On adding H2SO4 to water the resulting aqueous solution gets corrosive.
Reason: Hydronium ions are responsible for corrosive action.
Assertion : Phenolphthalein gives pink colour in basic solution.
Reason : Phenolphthalein is a natural indicator.
Assertion: HCl gas does not change the colour of dry blue litmus paper.
Reason: HCl gas dissolves in the water present in wet litmus paper to from H+ ions.
Assertion : HCl produces hydronium ions (H3O+) and chloride ions (Cl-) in aqueous solution.
Reason : In presence of water, bases give H+ ions.
Assertion: Sodium hydroxide reacts with zinc to produce hydrogen gas.
Reason : Acids react with active metals to produce hydrogen gas.
Assertion : Ammonia solution is an alkali.
Reason : Ammonia solution turns blue litmus paper red.
Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.
Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A) : The acid must always be added to water with constant stirring.
Reason (R) : Mixing of an acid with water decreases the concentration of H+ ions per unit volume.
|
85 videos|437 docs|75 tests
|