Test: First Law of Thermodynamics - NEET MCQ
10 Questions MCQ Test Physics Class 11 - Test: First Law of Thermodynamics
Suppose we have a box filled with gas and a piston is also attached at the top of the box.What are the ways of changing the state of gas (and hence its internal energy)?
Which of the following statement is true for a thermodynamical system where ∆U is the increase in internal energy and ∆W work done respectively?
Hot coffee in a thermos flask is shaken vigorously, considering it as a system which of the statement is not true?
The internal energy and the work done by a system decreases by same amount then
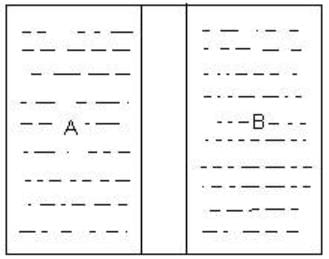
Which wall would allow the flow of thermal energy between systems A and B to achieve thermal equilibrium?
The first law of thermodynamics
1. Is a restatement of the principle of conservation of energy as applied to heat energy
2. Is the basis for the definition of internal energy
3. Is basis for the definition of temperature
4. asserts the impossibility of achieving an absolute zero temperature.
Internal energy of a system increases by 60 J when 140 Jof heat is added to the gaseous system. The amount of work done would be:
|
97 videos|379 docs|103 tests
|





















