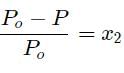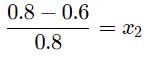Test: Colligative Properties (Lowering Of Vapour Pressure) - JEE MCQ
20 Questions MCQ Test - Test: Colligative Properties (Lowering Of Vapour Pressure)
This section contains 12 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
The vapour pressure of pure liquid solvent is 0.80 atm. When a non-volatile solute B is added to the solvent, its vapour pressure drops to 0.60 atm. Thus, mole fraction of the component B is
Vapour pressure of pure water at 298 K is 23.8 torr. Thus, vapour pressure of a solution containing 6 g urea and 68.4 g sucrose in 10 moles of water is
Vapour pressure of nitrobenzene (molar mass = 123 g mol-1) is 3.6 kNm-2 and that of water (molar mass = 18 g mol-1) is 977 kNm-2. They form immiscible mixture at the given temperature. Thus, percentage of nitrobenzene in the vapour phase is
The vapour pressure of n-hexane at 350 K is 840 torr and that of cyclohexane is 600 torr. Mole fraction of hexane in the mixture that boils at 350 K and 1 atm pressure assuming ideal behaviour is
At 298 K, the vapour pressure of pure water is 23.76 mm Hg and that of an aqueous solution is 22.98 mm Hg. Thus, molality of solution is (assume dilute solution)
A binary liquid solution is prepared by mixing n-heptane and ethanol. Which one of the following statements is/are correct regarding the behaviour of the solution?
18 g of glucose (C6H12O6 ) is added to 178.2 g of water. The vapour pressure of water for this aqueous solution at 100°C is
Which of the following liquid pairs shows a positive deviation from Raoult’s law?
A solution of two liquids boils at a temperature more than the boiling point of either of them. Hence, the binary solution shows
Lowering of vapour pressure of an aqueous solution of a non-volatile non-electrolyte 1 molal aqueous solution at 100°C is
Which combination of following terms is matched correctly?
I. Vapour pressure
II. Intermolecular forces
III. Latent heat of vaporisation
The solubility of urea in methanol is 17 g urea/100 mL methanol. Density of methanol is 0.792 g/mL at 293 K at which vapour pressure of methanol is 96.0 mm of Hg. Thus, vapour pressure of saturated solution of urea in methanol is
One or More than One Options Correct Type
This section contains 3 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Benzene and naphthalene form an ideal solution at room temperature. For this process, the true statement(s) is/are [JEE Advanced 2013]
Total vapour pressure of mixture of 1 mole of a volatile component A (p°A = 100 mm Hg) and 3 moles of volatile component B(p°B = 60 mm Hg) is 75 mm Hg. For such case,
At 35°C, the vapour pressure of carbon disulphide (CS2)is 512 mm Hg and that of acetone (CH3COCH3)is 344 mm Hg. A solution of CS2 and CH3COCH3 in which mole fraction of CS2 is 0.25 has a total vapour pressure of 600 mm Hg. Which of the following statement(s) is/are correct?
Comprehension Type
This section contains 2 paragraphs , each describing theory, experiments, data, etc. four questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage I
At 300 K, vapour pressure of pure liquid A = 575torr
and that of pure liquid B = 390torr
In binary mixture of liquids A and B with ideal behaviour, mole fraction of A in vapour phase =0.35
Q. Binary mixture contains
Passage I
At 300 K, vapour pressure of pure liquid A = 575torr and that of pure liquid B = 390torr In binary mixture of liquids A and B with ideal behaviour, mole fraction of A in vapour phase =0.35
Q. Total vapour pressure of the binary mixture is
Passage II
At 40°C, the vapour pressure (in torr) of methanol-ethanol solution is represented by
where, is the mole fraction of methanol.
Q. Difference of vapour pressures of volatile liquids (methanol-ethanol) in pure state at 40°C is
Passage II
At 40°C, the vapour pressure (in torr) of methanol-ethanol solution is represented by
where, is the mole fraction of methanol.
Q.
What is the mole fraction of methanol in vapour phase in a binary mixture with mole fraction of ethanol 0.50?
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct
100 g of binary mixture of A and B contains 60% of A. Mixture is subjected to the following operation.
Original mixture I II containing vapours of A and B
containing vapours of A and B
Match the content of Column I with their values in Column II and select the answer from the codes given below.