Test: JEE Main 35 Year PYQs- Some Basic Concepts of Chemistry - JEE MCQ
17 Questions MCQ Test - Test: JEE Main 35 Year PYQs- Some Basic Concepts of Chemistry
In a compound C, H and N atoms are present in 9 : 1 : 3.5 by weight. Molecular weight of compound is 108. Molecular formula of compound is [2002]
With increase of temperature, which of these changes? [2002]
Number of atoms in 558.5 gram Fe (at. wt. of Fe = 55.85 g mol–1) is
What volume of hydrogen gas, at 273 K and 1 atm. pressure will be consumed in obtaining 21.6 g of elemental boron (atomic mass = 10.8) from the reduction of boron trichloride by hydrogen ? [2003]
25ml of a solution of barium hydroxide on titration with a 0.1 molar solution of hydrochloric acid gave a litre value of 35ml. The molarity of barium hydroxide solution was [2003]
6.02 × 1020 molecules of urea are present in 100 ml of its solution. The concentration of urea solution is [2004] (Avogadro constant, NA = 6.02 × 1023 mol–1)
To neutralise completely 20 mL of 0.1 M aqueous solution of phosphorous acid (H3PO3), the value of 0.1 M aqueous KOH solution required is [2004]
The ammonia evolved from the treatment of 0.30 g of an organic compound for the estimation of nitrogen was passed in 100 mL of 0.1 M sulphuric acid. The excess of acid required 20 mL of 0.5 M sodium hydroxide solution for complete neutralization. The organic compound is [2004]
Two solutions of a substance (non electrolyte) are mixed in the following manner. 480 ml of 1.5 M first solution + 520 ml of 1.2 M second solution. What is the molarity of the final mixture ?[2005]
If we consider that 1/6, in place of 1/12, mass of carbon atom is taken to be the relative atomic mass unit, the mass of one mole of the substance will [2005]
How many moles of magnesium phosphate, Mg3(PO4)2 will contain 0.25 mole of oxygen atoms? [2006]
Density of a 2.05M solution of acetic acid in water is 1.02 g/mL. The molality of the solution is [2006]
The density (in g mL–1) of a 3.60 M sulphuric acid solution that is 29% H2SO4 (molar mass = 98 g mol–1) by mass will be
In the reaction, [2007]
2Al(s) + 6HCl(aq) → 2Al3+ + (aq) + 6Cl- (aq )+ 3H2 (g)
Consider the following reaction :
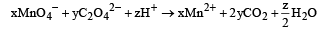
The value’s of x, y and z in the reaction are, respectively : [JEE M 2013]
A gaseous hydrocarbon gives upon combustion 0.72 g of water and 3.08 g of CO2. The empirical formula of the hydrocarbon is : [JEE M 2013]
Experimentally it was found that a metal oxide has formula M0.98O. Metal M, present as M2+ and M3+ in its oxide.Fraction of the metal which exists as M3+ would be : [JEE M 2013]


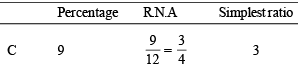
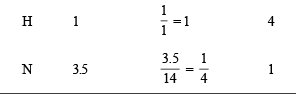
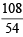 = 2
= 2
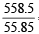 = 10 moles
= 10 moles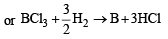
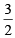 x 22.4 L at N.T.P
x 22.4 L at N.T.P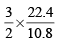 x 21.6 = 67.2 L at N.T. P..
x 21.6 = 67.2 L at N.T. P..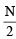 ⇒ M = 0.07 M
⇒ M = 0.07 M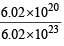
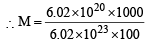 = 0.01M
= 0.01M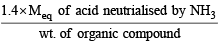
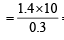 = 46.6
= 46.6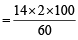 = 46.6
= 46.6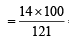 = 11.5% [C6H5CONH2 = 121]
= 11.5% [C6H5CONH2 = 121]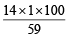 = 23.4% [ CH3CONH2 = 59]
= 23.4% [ CH3CONH2 = 59]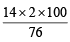 = 36.8% [NH2CSNH2 = 76]
= 36.8% [NH2CSNH2 = 76]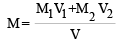 where V = V1 + V2
where V = V1 + V2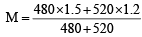 = 1.344 M
= 1.344 M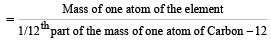
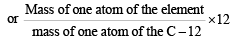
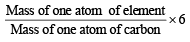
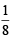 x mole of Mg3(PO4)2
x mole of Mg3(PO4)2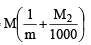
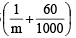
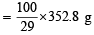 of solution = 1216 g of solution
of solution = 1216 g of solution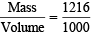 = 1.216 g/ml = 1.22 g/ml
= 1.216 g/ml = 1.22 g/ml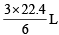 of H2 at S.T..PP
of H2 at S.T..PP 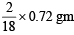 = 0.08 gm H
= 0.08 gm H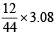 = 0.84 gm C
= 0.84 gm C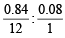 = 0.07 : 0.08 = 7 : 8
= 0.07 : 0.08 = 7 : 8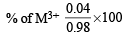 = 4.08%
= 4.08%










