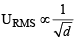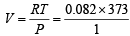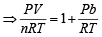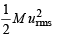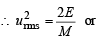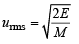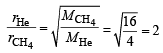JEE Advanced (Single Correct MCQs): States of Matter - JEE MCQ
30 Questions MCQ Test - JEE Advanced (Single Correct MCQs): States of Matter
Equal weights of methane and oxygen are mixed in an empty container at 25ºC. The fraction of the total pressure exerted by oxygen is (1981 - 1 Mark)
The temperature at which a real gas obeys the ideal gas laws over a wide range of pressure is (1981 - 1 Mark)
The ratio of root mean square velocity to average velocity of a gas molecule at a particular temperature is (1981 - 1 Mark)
Helium atom is two times heavier than a hydrogen molecule.At 298 K, the average kinetic energy of a helium atom is
(1982 - 1 Mark)
Equal weights of methane and hydrogen are mixed in an empty container at 25ºC. The fraction of the total pressure exerted by hydrogen is : (1984 - 1 Mark)
Rate of diffusion of a gas is : (1985 - 1 Mark)
The average velocity of an ideal gas molecule at 27ºC is 0.3 m/sec. The average velocity at 927ºC will be: (1986 - 1 Mark)
In van der Waals equation of state for a non-ideal gas, the term that accounts for intermolecular forces is (1988 - 1 Mark)
A bottle of dry ammonia and a bottle of dry hydrogen chloride connected through a long tube are opened simultaneously at both ends the white ammonium chloride ring first formed will be (1988 - 1 Mark)
The values of van der Waals constant ‘a’ for the gases O2, N2, NH3 and CH4 are 1.360, 1.390, 4.170 and 2.253 L2 atm mol–2 respectively. The gas which can most easily be liquified is : (1989 - 1 Mark)
The density of neon will be highest at (1990 - 1 Mark)
The rate of diffusion of methane at a given temperature is twice that of a gas X. The molecular weight of X is (1990 - 1 Mark)
According to kinetic theory of gases, for a diatomic molecule (1991 - 1 Mark)
At constant volume, for a fixed number of moles of a gas the pressure of the gas increases with rise in temperature due to (1992 - 1 Mark)
Longest mean free path stands for : (1995S)
Arrange the van der Waals constant for the gases : (1995S)
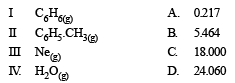
The ratio between the root mean square speed of H2 at 50 K and that of O2 at 800 K is, (1996 - 1 Mark)
X mL of H2 gas effuses through a hole in a container in 5 seconds. The time taken for the effusion of the same volume of the gas specified below under identical conditions is : (1996 - 1 Mark)
One mole of N2O4(g) at 300 K is kept in a closed container under one atmosphere. It is heated to 600 K when 20% by mass of N2O4 (g) decomposes to NO2(g).The resultant pressure is : (1996 - 1 Mark)
The compressibility factor for an ideal gas is (1997 - 1 Mark)
A gas will approach ideal behaviour at (1999 - 2 Marks)
The rms velocity of hydrogen is 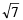 times the rms velocity of nitrogen. If T is the temperature of the gas, then (2000S)
times the rms velocity of nitrogen. If T is the temperature of the gas, then (2000S)
The compressibility of a gas is less than unity at STP.Therefore, (2000S)
At 100°C and 1 atm, if the density of liquid water is 1.0 g cm–3 and that of water vapour is 0.0006 g cm–3, then the volume occupied by water molecules in 1 litre of steam at that temperature is (2000S)
The root mean square velocity of an ideal gas at constant pressure varies with density (d) as (2001S)
Which of the following volume (V ) - temperature (T ) plots represents the behaviour of one mole of an ideal gas at one atmospheric pressure ? (2002S)
When the temperature is increased, surface tension of water (2002S)
Positive deviation from ideal behaviour takes place because of(2003S)
The root mean square velocity of one mole of a monoatomic gas having molar mass M is ur.m.s.. The relation between the average kinetic energy (E) of the gas and ur.m.s. is (2004S)
The ratio of the rate of diffusion of helium and methane under identical condition of pressure and temperature will be(2005S)


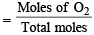
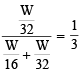

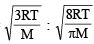
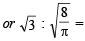 = 1.086 : 1
= 1.086 : 1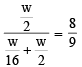
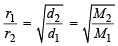
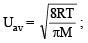
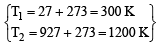
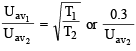
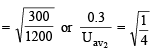
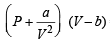 = RTT; Here
= RTT; Here  represents
represents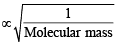
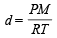
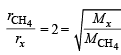
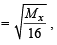 or Mx = 64
or Mx = 64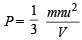 ...(1)
...(1)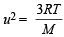 [explained from (1)]
[explained from (1)]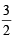 KT; Hence (d) is true.
KT; Hence (d) is true.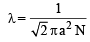
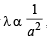 where a = molecular diameter
where a = molecular diameter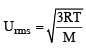
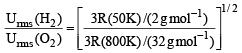 = 1
= 1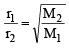
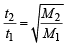
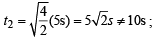
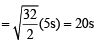
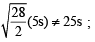
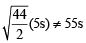
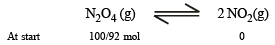
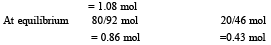

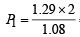 = 2.38 atm.
= 2.38 atm. 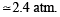
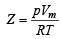
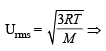
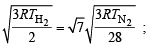
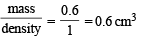
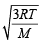 Using ideal gas equation,
Using ideal gas equation,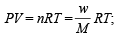
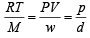 where d is the
where d is the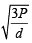 at constant pressure,
at constant pressure,